U.S. Authorities Seize $76 Million in Illegal Vapes
The FDA says it is collaborating with the federal task force to accelerate further enforcement actions
The FDA says it is collaborating with the federal task force to accelerate further enforcement actions

Most are believed to originate from the northern part of the island, beyond the republic’s control.

Any rise in the cost of vapes could drive smokers back to cigarettes, says Smoke Free Sweden.

Once individuals switch from tobacco smoking to vapes, there is no clear baseline for before and after use comparison.

The southeast Asian nation spends nearly 1.5 percent of its GDP treating tobacco-related diseased.

Revenue has suffered from a shift toward vaping and rampant illicit trade in tobacco products.

The Texas city is hosting launch events ahead of the bestselling heating product’s U.S. debut.

Three leading cigarette makers will pay $23.6 million under a mediator’s proposed plan.

The nation should step up its fight against tobacco, and make the industry pay, says activists.

The U.S. Supreme Court is scheduled to hear the case in early December.

But despite a pending marketing application, it is unclear whether the FDA will let Velo stay on the market.

Only 1.4 percent of students reported current cigarette smoking in 2024, according to the NYTS

Presentations highlighted new opportunities to accelerate harm reduction, including those involving genomics and artificial intelligence.

Some researchers say flavors encourage ‘initiation and experimentation.’

The TPA argues that the FDA’s actions have been detrimental to public health.

Earlier this week, the country’s president announced a ban on the sale, advertising and use of vapes.

Click on a country to view its latest news from the tobacco and vapor industries.

Efficiency will be the key to decarbonizing the EU transportation sector by 2050.

Tobacco companies are slowly gaining their footing in the cannabis business.
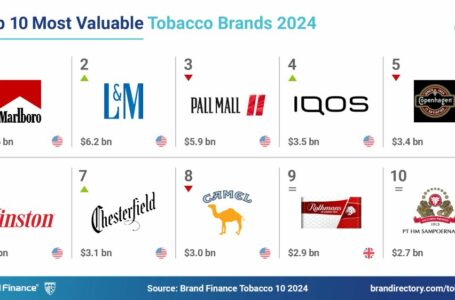
Marlboro remains the world’s most valuable tobacco brand, but cigarette alternatives are gaining ground.

With normal supplies cut off due to the Hamas-Israel conflict, cigarettes are selling for $30 per stick in Gaza.


There are still more unknowns than knowns about the shape of future European regulation for novel nicotine products.

Due to its versatility, homogenized tobacco remains key to tobacco companies’ operations.


Despite changing attitudes, FOREST still has an important role to play, says Director Simon Clark.

Recipients of the 2024 Golden Leaf Awards accept their trophies during the Global Tobacco and Nicotine Forum in Athens.


In Tobacco Reporter’s October 2024 issue, Cheryl Olson reflects on how the entry of heated tobacco might change the U.S. market. Due to a combination of regulation and an intellectual property dispute, American smokers have been deprived of tobacco-heating products that have become ubiquitous in many other markets.
As Philip Morris International prepares to (re)introduce its IQOS device there, it’s worth examining the challenges and opportunities facing heated tobacco in the world’s most lucrative tobacco market.
Barnaby Page examines the EU position on novel nicotine products as the trade bloc crafts a new Tobacco Products Directive, and George Gay explores how flexible packaging can be used to help manufacturers in responding to the EU’s nascent Packaging and Packaging Waste Regulations.
Vladislav Vorotnikov looks at the impact of war on the tobacco trade in Gaza, and Brand Finance unveils its latest ranking of the most valuable tobacco brands.
Also in this issue: The 2024 recipients of Tobacco Reporter’s Golden Leaf Awards.

The end of ‘Chevron deference’ doctrine could have ripple effects across the entire nicotine industry in the United States.

The critical role of site visits and quality controls in behavioral science research

Tabaterra wants to lift Azerbaijani tobacco to global quality standards.

The GTNF, scheduled Sept. 24–26 in Athens, is made possible by the generous support of these sponsors.

Having firmly established itself as a regional player, Tabaterra of Azerbaijan is preparing to take its operations to the next level.

With its plastic-free products goal at the forefront, Filtrona’s new filter technology allows for a sustainable RYO option.

Will the next EU Tobacco Products Directive embrace harm reduction?

Innovation, integrity and excellence remain at the core of SWM’s business under the company’s new ownership.

Philip Morris International is getting ready to reintroduce IQOS in the US.

Research institutions can carry out surveys that nicotine companies may hesitate to conduct themselves.

Broughton’s Malcom Saxton explains how to develop effective heated-tobacco products.

What we can learn from the first FDA marketing order for menthol ENDS, according to Broughton CEO Chris Allen.