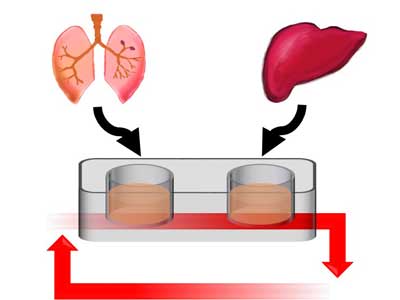
Researchers at Philip Morris International (PMI) have successfully connected human tissues from the lung and liver in a novel multi-organ-on-a-chip (MOC) in vitro model for toxicity testing. The lung-liver MOC has the potential to reduce the necessity for animal testing in the toxicological assessment of airborne toxins, facilitate the discovery of important mechanisms underlying diseases, and further the development of safer and more effective therapeutics. Details of the lung-liver MOC have been presented at the recent World Congress on Alternatives and Animal Use in the Life Sciences in Seattle, Washington, USA.
Researchers at Philip Morris International (PMI) have successfully connected human tissues from the lung and liver in a novel multi-organ-on-a-chip (MOC) in vitro model for toxicity testing. The lung-liver MOC has the potential to reduce the necessity for animal testing in the toxicological assessment of airborne toxins, facilitate the discovery of important mechanisms underlying diseases, and further the development of safer and more effective therapeutics. Details of the lung-liver MOC have been presented at the recent World Congress on Alternatives and Animal Use in the Life Sciences in Seattle, Washington, USA.
The growth of human tissue in two-dimensional models is now frequently replaced with three-dimensional (3D) modelling, which more accurately represents human biology. Microengineering now also enables the incorporation of small devices into 3D models to reproduce the complex microenvironments of specific organs. These are often referred to as organs-on-chips (OoCs). However, OoCs can only represent a single organ, and therefore cannot be used to study the many organ-to-organ interactions observed in the human body. The lung-liver MOC aims to address this by creating an accurate model of the lung and liver combined.
“Adding relevant liver tissues to in vitro models of the lung is essential for the thorough toxicological assessment of airborne compounds,” said David Bovard, post-doctoral fellow, systems toxicology, PMI. “While these compounds are absorbed through the lung, the true toxicity of some will only be realized following their metabolization by the liver. Combining lung and liver tissues effectively is very challenging, and we are delighted to have reached this important milestone. We can now begin to envisage an in vitro toxicity testing strategy that accurately represents the complexity of the human body and the interplay between organs, without the need for animal testing.”
The lung-liver MOC has been demonstrated to hold both lung and liver tissues in a stable state for at least four weeks. In order to assess whether the liver tissues used in the model would accurately metabolize, the activity of key enzymes that are known to be essential to the metabolization of medications was measured. In addition, the researchers measured the formation of metabolites following exposure of the liver tissues to nicotine and nicotine-derived nitrosamine ketone (NNK), one of the key carcinogenic components of tobacco smoke. The major nicotine and NNK metabolites normally found in smokers were detected, confirming the metabolic capacity of the liver tissues.
Not only do MOCs have the potential to reduce the need for animal testing, they may also provide more accurate, detailed and timely data. By using human tissues, MOCs avoid the challenges involved in translating results observed in one species to another. They may also unlock new approaches to drug development by providing an improved understanding of dose responses, enabling the detection of drug resistance, and highlighting potential side effects. In association with computational modelling to transform in vitro data into in vivo predictions, MOCs will also help in decreasing the rate of clinical failure of new drugs, which is estimated to cost between $800 million and $1.4 billion for anticancer drugs alone.
The lung-liver MOC is just one new organ-on-a-chip technology being developed by PMI. Also presented at the World Congress on Alternatives and Animal Use in the Life Sciences were details of an innovative 3D vasculature-on-a-chip. Developed in collaboration with MIMETAS5, the 3D vasculature-on-a-chip uses a microfluidic culture device to create a life-like blood vessel model. Researchers have successfully used this model to measure the attachment of immune cells to the blood vessel wall, a feature of early onset atherogenesis. As with the lung-liver MOC, the aim of the 3D vasculature-on-a-chip is to improve the prediction of the effects of intoxicants and therapeutic drugs in humans, while reducing the use of laboratory animals.