Encouraging results for carbon heated tobacco as an alternative to cigarettes
New data from three studies have demonstrated that the aerosol generated by Philip Morris International’s (PMI) carbon heated tobacco product (CHTP) has a significantly reduced biological impact as compared with cigarette smoke, according to PMI.
Two of the studies are pre-clinical, assessing the impact of the CHTP aerosol on human tissues using advanced in vitro models. The third is a randomized and controlled clinical study, investigating the reduction of exposure to harmful and potentially harmful constituents (HPHCs) after switching from cigarette smoking to using a CHTP.
The studies have been presented at the Congress of the European Societies of Toxicology, Bratislava, Slovakia, and the World Congress of Alternatives and Animal Use in the Life Sciences, Seattle, Washington, USA.
CHTP is a novel, smoke-free tobacco product that uses a carbon heat source to heat a tobacco plug to a controlled temperature not exceeding a well-defined threshold. This process prevents combustion of the tobacco plug and, at the same time, generates a nicotine-containing aerosol with tobacco flavor. By eliminating combustion of the tobacco, which occurs during use of cigarettes, CHTP has the potential to reduce the risk of smoking-related disease and adverse health outcomes.
“Not only do these studies add to the growing body of evidence suggesting that CHTP use may be less harmful than cigarette smoking, they also demonstrate what can be achieved with modern toxicological techniques,” said Julia Hoeng, director of systems toxicology, PMI.
“The studies adopt the widely recognized 3Rs principles—replace, reduce, refine—aimed at minimizing animal use, and we have shown how meaningful data can be acquired using in vitro based methodologies in combination with computational toxicology. As well as providing the potential to replace animal testing, these techniques can also offer more timely results, cost-efficiencies, and a deeper understanding of the biological processes underling toxicity.”
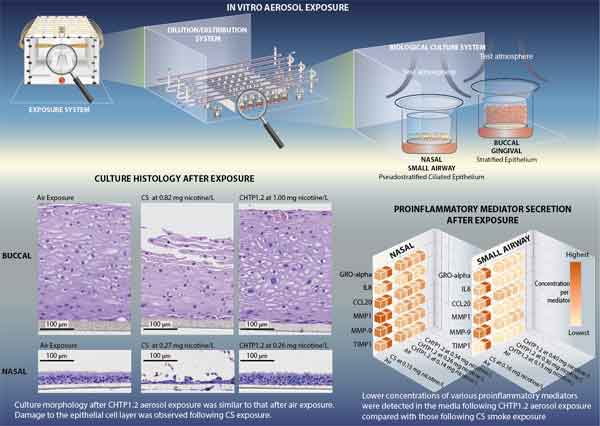
The two pre-clinical studies were conducted using three-dimensional cellular models developed from the primary epithelial cells of human donors, taken from the gums, the nasal cavity, and the small airway. Such models, which are developed using advanced tissue engineering techniques, have been shown to represent human biology more accurately than more traditional two-dimensional models.
The cultures were exposed to increasing concentrations of CHTP aerosol or cigarette smoke. Each concentration level of CHTP aerosol and cigarette smoke contained comparable nicotine concentrations. The impact of these exposures were assessed from a set of biological endpoints including cell morphology, cell toxicity, inflammatory responses, and global gene and microRNA expression changes.
Computational analyses were then applied to reveal further insights into the biological mechanisms at work and to make robust predictions of toxicity. In all cell types, and in all measured endpoints, CHTP aerosol exposure exerted a significantly lower impact than cigarette smoke.
The clinical study was designed to understand whether switching to CHTP reduces exposure to HPHCs. Eighty healthy adult smokers were randomized into one of two groups, either a) switching to use of CHTP, or b) to continued cigarette smoking. Both groups were assessed for five days in a controlled and confined setting.
The exposure to 15 HPHCs in cigarette smoke was measured using biomarkers of exposure in blood and urine (selected HPHCs were representative of various toxicity classes as defined by the FDA). After five days, levels of biomarkers of exposure to HPHCs were reduced in the CHTP group by 55.6 percent to 97.1 percent (for, respectively, “total 1-hydroxyprene” and “1-aminonaphthalene,” both of which are carcinogens). Notably, a reduction in “urge-to-smoke,” together with adequate nicotine delivery, indicate that CHTP could be a suitable substitute for cigarettes for current adult smokers.
CHTP is one of a range of technologies being investigated in PMI’s portfolio of reduced-risk products (RRPs). Further information on CHTP and PMI’s RRP development and assessment program can be found at www.pmiscience.com.En