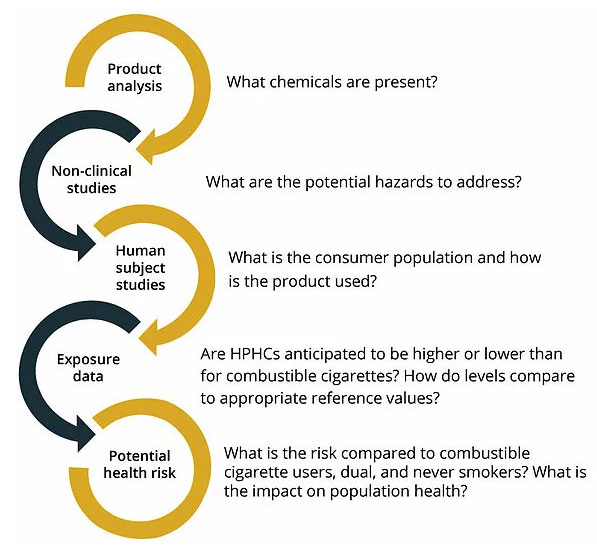
What’s new and why?
By Autumn Bernal
The U.S. Food and Drug Administration (FDA) has modified the list of constituents or chemicals of concern to be included on a Harmful and Potentially Harmful Constituent (HPHC) list. This guide outlines the chemicals that have been removed from the list, those that have been added, and considerations for aligning non-clinical, toxicology assessments with analytical testing for Electronic Nicotine Delivery Systems (ENDS).
Section 904e of the Food, Drug, and Cosmetics Act requires the FDA to establish and regularly define as appropriate a list of HPHCs. These HPHCs represent the FDA’s current thinking on which chemicals present in the consumable portion of a tobacco product are most representative of the health risks commonly linked to tobacco use: cancer, cardiovascular disease, respiratory effects, reproductive problems and addiction. Though the revised list has yet to be published in the Federal Register, the FDA recommends that companies analyze 33 chemicals, along with flavor constituents that may be respiratory irritantants, to support their Premarket Tobacco Product Application (PMTA) applications for ENDS.
What chemicals have been removed?
Eight chemicals have been removed from the list of HPHCs recommended for analysis in ENDS products. These include 4-aminobiphenyl, 1-aminonaphthalene, 2-aminonaphthalene, ammonia, anabasine, benzo[a]pyrene, 1,3-butadiene and isoprene. These chemicals are currently listed on FDA’s “Established List of Chemicals and Chemical Compounds Identified by FDA as Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke” (2012) and are classified as carcinogens, respiratory toxicants, addictive substances, and/or reproductive and developmental toxicants.
These chemicals have been removed as they are mainly considered to be related to the treatment or combustion of tobacco, and hence are considered less relevant for ENDS products.
What chemicals have been added?
The most notable changes to the list are the addition of 12 chemicals listed below, as well as nicotine from any source and flavorants, as applicable.
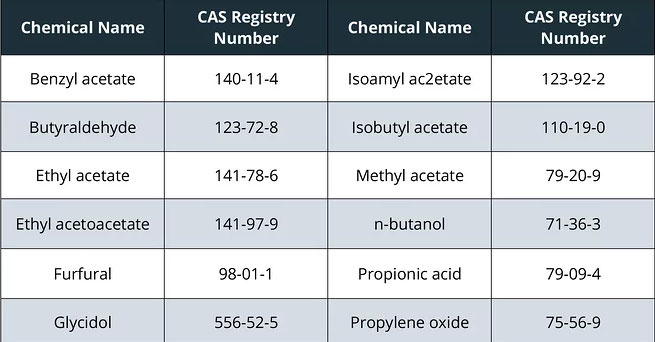
These additions are based on FDA’s current thinking that these chemicals could cause health hazards depending on the level, absorption, or interaction with other constituents. For example, propylene oxide is also included on the 2012 HPHC list as a carcinogen and respiratory toxicant. Although the FDA has not provided their own hazard classifications for the other newly listed chemicals, a closer look at these chemicals by reviewing publicly available toxicology databases indicates several potential health outcomes, such as respiratory system irritation.
And … remember to consider flavorants
The FDA guidance adds that manufacturers also consider whether to test for flavorants that can be respiratory irritants such as benzaldehyde, vanillin and cinnamaldehyde. This recommendation underscores the advantage of implementing a systematic toxicological screening assessment early in your PMTA planning to support an analytical testing strategy. Early toxicological screening during a planning phase will also steer the subsequent non-clinical studies proposed to support an evaluation of whether your product is appropriate for the protection of public health.
Do all the chemicals need to be tested?
The FDA explains in its guidance that it expect applicants will report the levels of HPHCs as appropriate for each product, so the reported HPHCs will differ among different product categories (i.e. liquid or hardware). A well thought out PMTA plan and consultation with FDA in a briefing meeting can provide clarification on product-appropriate testing. For example, for liquids, understanding product formulations, including flavorants, can provide justification for a client-specific HPHC testing plan. Also, the FDA recognizes that some of the constituents and chemicals listed may be ingredients in e-liquids (e.g. menthol, propylene glycerol, glycerol etc.). In these cases it may be acceptable to simply provide the quantity added, rather than measure constituent or chemical yields generated from the e-cigarette.
Dose is important when evaluating HPHCs
For PMTA submissions, complete toxicology profiles of the HPHCs will identify potential adverse health outcomes, the doses at which these potential effects are seen, and any exposure guidance values established by governmental agencies. Dose is important. As the FDA notes, the chemicals could cause health hazards “depending on the level.” Thus, any potential health effects should be considered in the context of the consumer exposure to determine potential health risks. This indicates the importance of product analysis of liquids and aerosols being closely aligned with both toxicological assessments and clinical evaluations of consumer use (human subject studies). Information necessary to evaluate HPHCs in the context of dose, and in comparison to other tobacco products and appropriate reference values, can then be provided.