Measuring Up
- Print Edition Science
- August 1, 2020
- 0
- 17 minutes read


How instrumentation suppliers and laboratory service providers are supporting customers with their PMTA submissions
By Stefanie Rossel
Although there has been a 120-day extension due to the outbreak of the Covid-19 pandemic, Sept. 9 will definitely be the final day: If a company wants its “recent” tobacco products to remain in the U.S. market, it has to submit a premarket tobacco product application (PMTA) to the U.S. Food and Drug Administration (FDA) by then. In 2016, the FDA announced that all tobacco products not on the market prior to Aug. 8, 2016, would require authorization before entering the market and all products on the market prior to that date would have a grace period during which companies could prepare their PMTAs for submission.
The candidate products will all undergo a several-stage review during which they have to demonstrate with the help of scientific data that they are “appropriate for the protection of public health.” The FDA will consider the risks and benefits of the products not only to the individual but also to the overall population, including whether existence of the product will increase the likelihood of nonusers starting using them. The evaluation also includes reviews of product ingredients, constituents, toxicological profile, health impact, manufacturing and packaging processes as well as labeling.
Tobacco Reporter asked several instrumentation suppliers and providers of laboratory services how they experienced the run-up to the FDA’s extended deadline, which was originally set for May 12, 2020.
“The date for the premarket submission was set well in advance, so there was increased demand but no real ‘rush,’” says Tobias Krebs, managing director of German company Vitrocell, which specializes in in-vitro testing technology.

Ian Tindall, head of innovation and marketing at Cerulean, says there has been a noticeable demand for extra testing capacity in the U.S. but that most of this demand was generated in late 2019, and the extension to the submission date had not really been visible.
“The larger companies have had this well organized all the way along, and from our experience, the anticipated rush for testing at the contact research organizations has not been mirrored in a late rush for test equipment,” Tindall explains.
“I should note that the EVALI crisis at the back end of 2019 did generate demand for additional testing of vaping products and a consequent surge in instrument demand, and this did put pressure on the business, but we feel we met all the urgent demands successfully. More recently, some of the focus has moved away from vaping devices towards tobacco-heating products, and we have been increasing supply kits and specialist machines and upgrades for this sector of the industry.”
In February 2019, Cerulean teamed up with Tews, a leading supplier in the field of industrial microwave moisture and density measurement. Cerulean now exclusively markets Tews laboratory devices for the tobacco industry. Based in Hamburg, Germany, Tews also has a U.S. subsidiary. “We can now jointly address emerging requirements for density and moisture testing in the industry and work effectively with clients in this regard,” says Tindall.

Thomas Schmidt, director of scientific and technical affairs at Borgwaldt KC, a German manufacturer of high-end quality control instruments and precise measurement devices that is part of the Hauni group of companies, notes that the PMTA process was communicated early and the instruments used to generate the data were available. “Besides new developments like our next generation of analytical vaping machines, the NGX series, we have, however, made some modifications to existing instruments—on our LM5SP and LM5SF, for example, which are both intended to collect aerosol in different collections procedures and increased the flexibility in usage to meet the specific demands of certain customers,” says Schmidt.
Broughton Nicotine Services (BNS) has noted a definite increase in demand for its services in the runup to the PMTA deadline, according to Chris Allen, vice president of scientific and regulatory affairs. For many companies, he contends, the PMTA has been the first opportunity to truly characterize and stress test their products.
“This has inevitably led to clients learning more about their products and the need to understand further so that risks can be mitigated and improvements made in the future,” Allen says. “The extension has provided additional time for these companies to perform further characterization and bolster their applications. In addition, we’ve seen a wave of companies who had been delaying the PMTA process or having partial information take advantage of the extension in order to commit to the PMTA pathway.”

Heavy workload
Matters are complicated by the FDA’s requirement that applicants file a separate application for each brand variant. For suppliers of instrumentation equipment and labs, this has meant a lot of additional work in recent months. “This has had no impact on our business but has required software development to become FDA compliant,” says Eric Favre, managing director of Sodim, a French company specializing in metrology for the tobacco industry and, like Borgwaldt KC, part of the Hauni group of companies. Sodim has developed a specific device called VPA (vaping puff analyzer). “This device has required adaptations for new formats with specific sample holders,” says Favre.
“The established manufacturers of traditional tobacco products have lots of data available for marked products, which they can provide to the FDA. In [the] case of modified and new products, especially vaping products, the manufacturers were not all aware of what is expected,” Schmidt points out. “These products are newer to the market and do not have sufficient historical data available to refer to. Therefore, we were not surprised that this has led to an increased demand for our next-generation product (NGP) machines and has driven us to develop a new generation of analytical vaping machines, our NGX family and other innovations and modifications within the available Borgwaldt KC portfolio to meet the needs of the market.”
Allen says that although each product is unique, the FDA is accepting “bundled” submissions for multiple products, which enables clients to save cost and tell the “story” of a group of products. “Despite the ability to include multiple products under one submission, the level of analytical testing has been significant,” he says. “We had been planning our expansion in line with the PMTA since 2015 and moved into our new dedicated nicotine products facility in 2018.”
The expansion project was completed in January of 2019 following an investment of £10 million ($12.41 million) into people, facilities, analytical equipment, software development and quality standard certification. The company recruited more than 70 new team members, not only within the analytical function but also across clinical, nonclinical and project management.
Vitrocell observed increased demand for systems with higher output. “We needed to increase our development activities but could tie in new orders in our regular production scheme,” says Krebs.
“We are fortunate that in the way we have set up our supply chain and manufacturing facility we can redeploy resources quickly and keep customers satisfied with extending lead times,” Tindall says. “Some special requests have taken a little longer to fulfill than our standard lead time, but by switching capacity, say, from our standard smoking machines to vaping and THP testing machines, we have managed to meet our delivery commitments. That is not to say we have not met bumps in the road, but the unsung heroes in the back office who keep the production machine running have really risen to the challenge.”
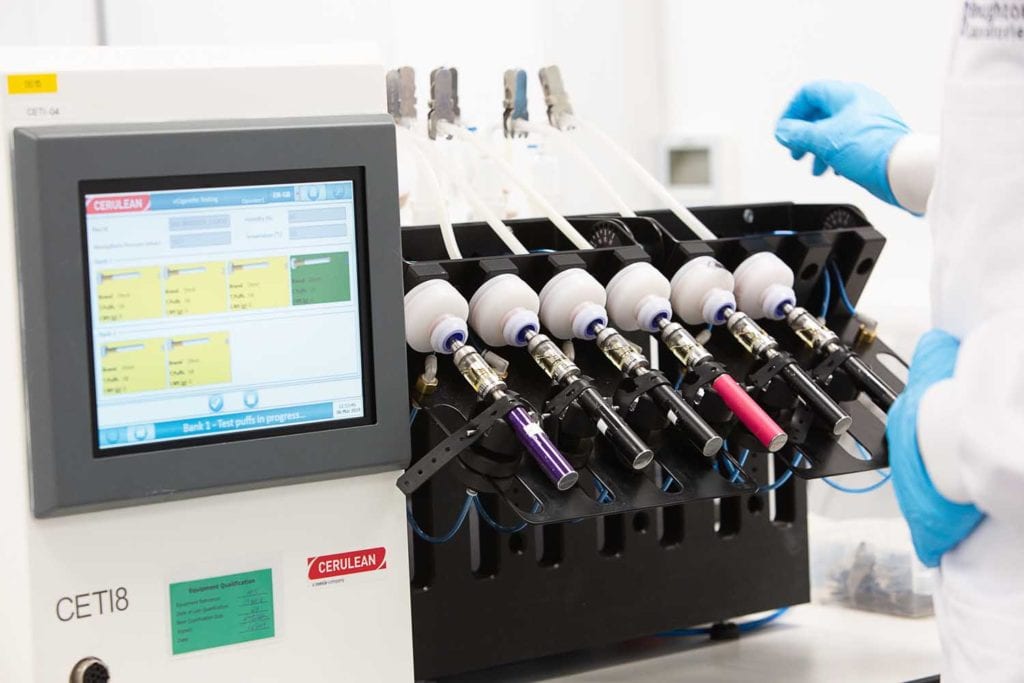
Far-reaching measurements
Testing required for PMTA applications is manifold; it includes the analysis of tobacco and e-liquid constituents, ingredients and additives, especially the 33 substances listed by the FDA as harmful and potentially harmful constituents (HPHCs), smoke and vapor constituents as well as all physical parameters of the products, toxicological assessments and topographic data, notes Schmidt. “We have seen a remarkable increase in interest in our topography products as well as our NGP vaping devices,” he says.
In vitro data play a role in the application too. For Vitrocell, this translated into demand for its exposure solutions. Cerulean witnessed greater interest in its vaping machines, particularly with an accessory that determines the density of the vapor. “We have published a lot of work that shows how this device, primarily designed for showing when an e-cigarette stops effectively forming aerosol, can be used to monitor the full life delivery of aerosol of an e-cigarette device,” Tindall explains. “Beyond measurement is the need from our customers’ perspective for installation and servicing in line with good laboratory practice [GLP], with an installation qualification and an operational qualification step that is properly documented.”
According to Allen, the level of testing required to demonstrate the shelf life of the product, including extractables and leachables, should not be underestimated. “Having the analytical data available is just the start of the process. Conclusions need to be drawn from this data to understand the performance of the product and most importantly to risk assess from a toxicological perspective,” he says. “This is why we saw the creation of the integrated chemistry consultancy and toxicological teams of paramount importance as having these teams on-site enables us to design the analytical studies in line with the end purpose and perform ‘real-time’ risk assessments.” BNS has also developed ToxHQ, a unique internal software tool to complete rapid toxicological screening and risk assessment. The central repository holds chemical data used in e-liquid formulations, including chemical identifiers, properties and classifications, such as HPHC-registered chemicals, Allen says.

Managing the data
Once the products have been authorized, further measurements will be needed to maintain compliance. “This will be an interesting development over the coming months and as we start to see FDA feedback on the PMTAs,” Allen points out. “It will very much depend upon the product sub-category, e.g., e-liquid, closed system or open device, but the critical point is demonstrating that you have control over the finished product. A key part of the PMTA is to understand how your product behaves, what the risks are and therefore what quality control checks need to be in place as either verification of incoming parts or ingredients, in-process controls or analysis of the manufactured product.”
As an instrumentation supplier, Tindall says the company must recognize that once equipment has been bought, its use might change subtly over time. “We have a mission to ensure that time does not make our products obsolete. Consequently, as part of our product development strategy, we have upgrade paths plotted for most equipment that allows adaptations as regulatory pressures change or research applications and the new products by research demand. One thing beyond ongoing measurement that should not be minimized is the need to ensure that the equipment used is running properly.”
In the course of a PMTA as well as during the control period afterward, massive amounts of data are generated, requiring expert management. Borgwaldt KC’s instrumentation features a data collection tool for this purpose. The company offers a variety of methods for collection, storage and submission of data to the customer’s data management system. “Our products are in compliance with the FDA requirements related,” Schmidt says, “but in general, the quantitative analysis of specific substances is requiring other analytical equipment in the labs.”
Vitrocell offers solutions that enable its systems to be operated with GLP compliance. “Beyond that, data management is performed using the current technology of the customer,” Krebs relates.
“There is a lot of data generated, and we have worked with a number of customers to ensure that there is a seamless exchange of data with their laboratory information and management systems (LIMS),” Tindall states. “The vast number of LIMS suppliers has meant that we have had to do this on a case by case basis, and we feel we have been successful in this. We are very conscious of the increasing burden of data generation and storage. Our 21CFRpart11 packages, which are available on many of our products, create complex audit trails. The Internet of Things (IOT) and Industry 4.0 initiatives will increase the amount of data on machine performance that is available to an auditor, user or engineer, and this will be the next challenge to face.”
Allen says that the retention of records is critical within any regulatory process. As BNS evolved from its pharmaceutical facility Broughton Laboratories, retention of both hard and electronic data has always been an integral part of the company’s quality management system. “Over the past twelve months, we’ve generated in excess of 200,000 lines of HPHC data for our clients, so effective data management is key. The utilization of LabHQ LIMS, developed by our sister company Broughton Software, including integration with the laboratory instrumentation and dynamic reporting, enables us to manage such large datasets.”
The handful of PMTA approvals to date were granted after long and tedious reviewing processes. Thus far, only three products have received marketing authorization: Swedish Match’s General snus, Philip Morris’ IQOS device and Heatsticks along with two varieties of 22nd Century’s Moonlight cigarettes. What happens when the FDA gets overrun with submissions remains to be seen. Favre expects feedback from the FDA before the end of this year.
“There will be huge amounts of data for the regulators to review,” Schmidt says. “It will be a monumental task to say the least.”
“The FDA [is] undoubtedly going to see a significant number of applications,” Allen comments. “The PMTAs we’ve been working on consist of tens of thousands of pages, so this is going to be a large volume of data for [the] FDA to review. The big question will be how many PMTAs make it through to the review stage, which will impact most on [the] FDA’s resource. Once in substantive review, this very much depends on what additional data is requested by [the] FDA as any major amendment of an application, either by the applicant or at the FDA’s request, would result in a new 180-day review period.”
He concludes: “In the same manner that all regulated industries have had to evolve, the electronic nicotine-delivery systems market will be no different. The PMTA process is simply a starting point, and as the FDA learns more about the products and risks, we’re likely to see further guidance issued—for example, the addition of new analytes and a deeper understanding of other possible chemical reactions and their risks to human health.”


