Firms Eye Synthetics After FDA Rejections
- Featured News This Week
- August 31, 2021
- 0
- 1
- 3 minutes read
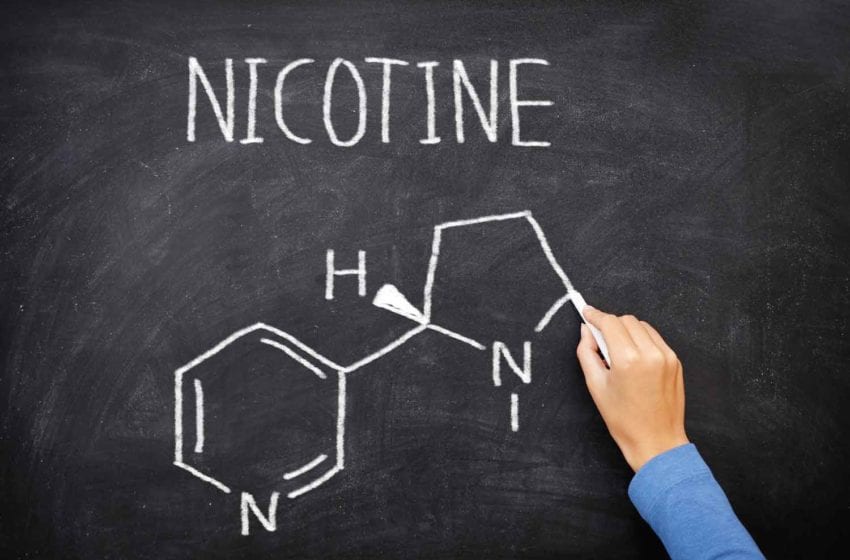
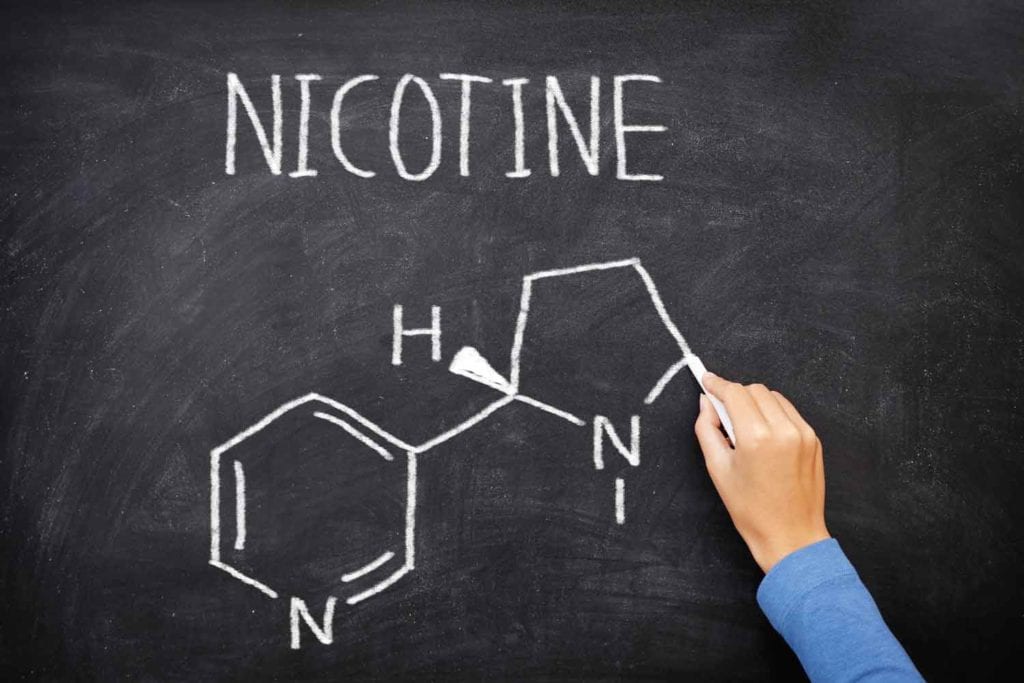
Vapor Salon will be switching to synthetic nicotine, the company wrote in a public Facebook post dated Aug. 26.
The post was published on the same day that the U.S. Food and Drug Administration denied some 55,000 marketing applications by Vapor Salon and two other companies on the ground that they “lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by well-documented, alarming levels of youth use of such products,” according to an FDA press release.
“VaporSalon is switching to TOBACCO FREE NICOTINE on Friday, 8/27/2021,” the Facebook post reads. “The main purpose of this is to be outside of the FDA’s regulations with their hefty PMTA requirement, which takes full effect on Sept. 9, 2021, with needing an approved PMTA, or your product can no longer be sold. There has been 0 approved PMTA’s for anything ENDS related to date.”
According to Filter, more manufacturers have begun looking at the possibility of synthetic nicotine as a way to avoid having to comply with FDA regulations.
The FDA defines a “tobacco product” as anything “made or derived from tobacco that is intended for human consumption, including any component, part or accessory of a tobacco product.”
Eric Lindblom, a senior scholar at Georgetown’s O’Neill Institute for National and Global Health Law and a former director of the FDA’s Center for Tobacco Products Office of Policy, said that, in response to such moves by vapor companies, the FDA could either assert jurisdiction over synthetic nicotine as a tobacco product or push for synthetic nicotine to be regulated like any other drug.
Because synthetic nicotine is more expensive than the natural variety, a switch would likely result in higher prices for consumers. Vapor Salon indicated that many of its redesigned products will now have “an upcharge,” according to Filter.
