Changing the Conversation
- Also in TR GTNF Print Edition
- November 1, 2023
- 0
- 23 minutes read


From Sept. 19 to Sept. 21, 2023, stakeholders from around the world gathered in Seoul to discuss the challenges and opportunities in the nicotine business, particularly as they relate to tobacco harm reduction. Attracting delegates from all parts of the globe, the event featured a wide variety of experts, including public health professionals, consumer advocates and financial analysts along with top regulators, such as the director of the U.S. Food and Drug Administration’s Center for Tobacco Products, Brian King. Below are highlights from the conference.

Keynote: Kingsley Wheaton (given by Jonathan Atwood)
During GTNF 2023, BAT’s global head of business communications, Jonathan Atwood, told attendees how BAT’s five-step plan for regulation could support achieving the right balance between harm reduction and the unintended consequences of access, including underage use.
“As an industry, we stand at an important crossroads. There is much confusion as to the way forward. Consumers are slightly confused. Doctors are slightly confused. Regulators are slightly confused and are struggling to enforce the laws they have written,” said Atwood. “What’s too often missed is the opportunity that tobacco harm reduction presents. The opportunity for a more progressive environment where both tobacco harm reduction and the role of [vaping products] is far better understood.”
Speaking on behalf of Kingsley Wheaton, BAT’s chief strategy and growth officer, Atwood said that reckless players in the market need to be penalized when they do not abide by the rules. He said the five suggestions are the areas that regulators should explore and then establish “smart regulation” that is right for their market.
“When I talk about smarter regulation, I mean regulation that is evidence-based, concentrated by nature, and achieving its policy aims while also avoiding unintended consequences. Greater partnership is required to achieve this,” Atwood said. “We must join forces externally with regulators and policymakers to try and create catalysts for positive change if smoke-free ambitions are to be met. Sustained and lasting changes to consumer behavior are difficult. However, it is consumer choice that offers the greatest hope for making a cigarette obsolete.”
Atwood said that the five areas were where smarter regulation could be applied to the vapor category to build a “more progressive environment” for tobacco harm reduction. He said the recommendations would need to be applied to the entire market and combined with greater enforcement. The five steps Atwood outlined included:
- On-device technology and functionality: Vapor products should be accessible only to adults. Both underage prevention and restriction is crucial. On-device technology, when applied and enforced across entire markets, could help in this regard.
- Flavors: More recognition is needed that flavors are an important driver of adoption for smokers seeking alternatives. However, flavors in vapor products should not particularly appeal to anyone underage.
- Manufacturing and import level: ensuring that noncompliant products cannot reach the market in the first place.
- Right to sell: Where no restrictions exist already, regulators may want to look at who should be able to sell vapor products and where. Reasonable safeguards at the point of sale would help ensure these products are sold only to adult consumers. Solutions such as retail licensing and facial recognition technologies should be seriously considered.
- Enforcement and penalties: Governments must wield their power and ensure consumers are purchasing legitimate products. Such measures should be rigorously enforced, and those who fail to comply should face meaningful sanctions.
Atwood said BAT was calling upon governments, regulators and industry peers to rally toward a sustainable and progressive environment in which vaping products are sold and marketed responsibly.
“The time for boldness is now. The time to change the conversation is now. The time to change the outcome is now. The opportunity for change is here. It is not about relaxing regulations. It’s about recalibrating them to align with the evidence and aspirations of millions seeking a better alternative to smoking,” said Atwood. “We have the opportunity to redefine the future of public health, and it begins with smarter regulation that reflects the reality of smoking alternatives and provides smokers the freedom to choose less risky products.”

Panel: Putting Consumers First
Toward the end of the Putting Consumers First panel held during September’s Global Tobacco and Nicotine Forum in Seoul, South Korea, Matt Drodge, research director at Walnut Unlimited, made the point that while nicotine consumers were all different, they all wanted to be able to make informed decisions about whether to continue smoking combustible cigarettes or when and how to make the transition to new nicotine products.
Of course, nicotine users can make such transitions only in countries where regulations allow them to do so, and the moderator of the panel, Nancy Loucas, public health policy expert and executive coordinator of the Coalition of Asia Pacific Harm Reduction Advocates (CAPHRA), made the point that the panelists represented countries forming a continuum of nicotine regulation.
Panelist Samrat Chowdhery, former president of the International Network of Nicotine Consumer Organisations, told participants that he felt unhappy about representing India, a country that had put consumers last by effectively banning vapes. Chowdhery said this is a pity because India does not have a strong or widely used public health network, so prevention, including through the use of safer alternatives, is vital, as it is in other parts of the developing world where 80 percent of tobacco users live.
Fiona Patten, leader of the Reason Party and former member of the Legislative Council of Victoria, who was unable to attend the GTNF in person and instead recorded a video message, apologized for representing Australia, a country that she said is leading the way on what not to do around tobacco harm reduction. Patten said that Australia’s “so-called medical model” of regulation is so onerous that 99 percent of Australians who are looking for a safer way to consume nicotine are being forced onto the black market.
Alex Clark, CEO of the Consumer Advocates for Smoke-Free Alternatives Association, who also did not attend the event, but appeared via a live link, said the premarket tobacco product application (PMTA) system in the U.S. appears to consumers to be acting as a very tight bottleneck on the products they have access to; no flavored products have been authorized through the system, only variations of tobacco. Beyond the PMTA system, there was also concern that a methodical state-by-state, municipality-by-municipality effort to severely restrict the availability of lower risk products would continue. Clark said that while he hopes that in the future people will be able to find products they can trust, he questioned why there has to be a delay. What is needed now is to disseminate the idea that nicotine users are not just data on a spreadsheet and to get that message out, elevating it up the chain to the regulator.
Clarisse Yvette Virgino, a Philippines-based member of the CAPHRA, had a more positive tale to tell because a “wild journey” that had seemingly been headed toward prohibition had ended with regulation. The regulations were somewhat burdensome, however. Retailers had a lot of rules to comply with, and there was a problem when it came to consumer choice because manufacturers had withdrawn certain products, such as juices, rather than go through the process of complying with what were stringent requirements.

Panel: Reinforcing Scientific Research
During the Reinforcing Scientific Research panel held as part of September’s Global Tobacco and Nicotine Forum in Seoul, South Korea, New Zealand-based Marewa Glover, director of the Center of Research Excellence on Indigenous Sovereignty and Smoking, made the point that the forum had heard many times how there is a need for tobacco and nicotine policies to be grounded in science and, therefore, evidence-based. There is, in fact, no disagreement on this point between tobacco control officials and tobacco harm reduction (THR) advocates, she said. However, care needs to be exercised because a form of evidence has been appropriated by some opposed to THR, and they are driving a broad social change agenda aimed at instituting a utopia where, for instance, no one would ever use drugs. They used scientific platforms loaded with people who shared their views to spread their ideology. They redefined the meanings of words so that unproven assertions became facts and facts became lies. To combat such views, she added, it is necessary for THR advocates to produce demonstrably robust research as part of a project that includes a communication strategy identifying stakeholders and how the information is to be gotten to them.
Glover had been asked three questions by moderator, Mark Littlewood, director general of the Institute of Economic Affairs: What needed to be done to reinforce scientific research into tobacco and nicotine?; who should form the audience for the research findings?; and how should the findings of junk science be challenged?—questions he also posed to the rest of the panel.
Kai-Jen Chuang, professor in the Department of Public Health at Taipei Medical University, explained that THR is not taught as part of medical degrees in Taiwan and therefore is not a well-recognized term, even though harm reduction principles are engaged in other areas. In fact, vapes were banned in Taiwan on March 22 this year. What is taught is health promotion and, more latterly as part of social-work courses, disaster reduction. He pointed out, nevertheless, that health promotion, disaster reduction and THR have similar goals, so those from outside Taiwan wishing to engage at conferences with public health officials over THR principles should present their papers, translated into Chinese, as health promotion studies. Nevertheless, he warned that it would be difficult, because of their training, to convince public health people of all stripes of the efficacy of using new technology to reduce the harm caused by smoking. The starting point for getting across messages about THR, he added, should be scholars with open minds, and from there, the focus could move to journalists and politicians.
Riccardo Polosa, professor of internal medicine at the University of Catania and founder of the Center of Excellence for the Acceleration of Harm Reduction (CoEHAR), said there is a need to reinforce quality science that has good repeatability. Repeatability, he added, is currently in crisis and not just in respect of tobacco control science, so the CoEHAR has established a comprehensive repeatability program pertaining to research into toxicity and biology in respect of combustion-free, nicotine-containing products. The program involves setting up and researching in seven laboratories with the same equipment and the same procedures to come up with super strong findings. Another thing that is needed, Polosa said, is to shift the focus from risk to harm. Relative risk has been studied for a decade now, and it is an easy win if combustible products are compared with combustible-free products. So now is the time to look at the absolute risk and show that it is low and that the level of harm is super low. This would provide a better position from which to convince governments, regulators and the public. Polosa had some good news on junk science, which he said is easy to debunk because it is junk, though this takes energy and time and requires a willingness to do it. A global network of scientists is actively rebutting junk science articles, though the challenge now is to speed up this process of rebuttal.
Picking up on an earlier comment about uncertainty, Konstantinos Farsalinos, research fellow at the Onassis Cardiac Surgery Center, made the point that uncertainty is inherent in science and essential to human progress. There is nothing wrong with uncertainty provided that it is not used to maintain the status quo, especially where the status quo has failed miserably. It is important, also, that uncertainties are not used as the basis for decision-making, which needs to be based on current knowledge. But one problem being faced today is that current knowledge is not being used to make decisions [about THR]; rather, decisions are being made on the back of the abuse of uncertainties [that the long-term use of THR strategies are unknowable at this stage]. Revisiting the 1986 Ottawa Charter for Health Promotion by the World Health Organization, Farsalinos said, it is obvious that it was basically talking about harm reduction in our everyday lives. Everything, even medicine itself, was a harm reduction science because it was not possible, probably, to cure any disease besides certain infections. We were treating diseases and reducing the adverse effects and consequences of diseases. This was all known. What is needed now is the reinforcement of the applicability of scientific data, of which there is a lot, on decision-making, something that has not happened in many parts of the world. Finally, Farsalinos said that he has had bad experiences in rebutting junk science even though he has been successful. Basically, he had run into a wall behind which people had decided what they believed and were casting around for the data to support their predetermined views. With science, you have to do the opposite of that, he said.

Keynote: Brian King
When Brian King speaks, people come to listen. The U.S. Food and Drug Administration’s Center for Tobacco Products (CTP) director’s keynote address was easily the best attended session of GTNF 2023. King’s speech served as an overview of the current state of the CTP and an outline of the center’s main priorities over the next few years.
King said that the CTP has made considerable progress in reducing combustible cigarette smoking in the United States, which he contends as one of the most remarkable public health achievements of the past century. He hopes that those declines continue, given that “we do know” that combustible smoking is responsible for the overwhelming burden of death from using combustible tobacco.
Tobacco use continues to cost the U.S. government a considerable amount of money—to the tune of $600 billion per year from both direct healthcare costs and lost productivity, according to King. He said there are important human health benefits as well as financial benefits for regulators to continue to focus on reducing combustible use in the United States. As a part of this focus, he said, the CTP is continuing to make inroads when it comes to premarket tobacco production application review.
“We have a new director of our Office of Science who has jumped in headfirst to continue to fiercely lead our 550-plus scientists on application review …. We have processed 99 percent of those and continue to finalize the remaining 1 percent. I’m hopeful that in the coming months and years, we will get back to what was intended to be a premarket approval process,” said King. “In the meantime, we have authorized 23 e-cigarettes, all tobacco flavored. So, it is possible. We have had successful authorizations. But again, I can’t reinforce enough the importance of providing that sound and robust science to inform on potential authorization.
“And it is possible, as you can see. There will be more authorizations in the future, but it’s important that we have that science to support those decisions. As I noted earlier, we also continue to fold in the nontobacco nicotine work into our broader portfolio around regulation. We did receive a million applications, which I don’t think anyone anticipated. I will say that we are making great numbers. We are 99.9 percent through with the review of those. I will say that 100 percent is very imminent.”
King said that when it comes to products that are illegally on the market (having received a marketing authorization and are not currently under review by the CTP), the CTP is mindful of the importance in exercising all authorities that it has to ensure that people are complying with the law. He said that the FDA has given retailers the information they need to comply with the law through a list of authorized products (the 23 products that have been authorized for sale). The CTP also continues to ramp up efforts in terms of training, education and outreach across the supply chain, particularly to retailers.
“We also continue to do surveillance inspection investigations. This is something that occurs on a daily basis. We have arrangements with all 50 states and territories to continue to do investigations. We have issued many warning letters for flavored disposable e-cigarettes, which we know are particularly popular for youth,” said King. “There’s been a variety of blitzes that have occurred monthly throughout the summer. I will say there are more to come. We are going to continue to conduct those blitzes and making sure that we are routinely monitoring, particularly with a focus on those products that we know have high youth appeal.
“On balance, we are also continuing to do work around issuing import alerts. I was a little tickled by all the attention that the import alert on Elf Bar got. That’s nothing new, folks. We’ve been doing that for many years. It was suggested it was something seismographic, but we’ve been doing import alerts for quite some time. And we do use those as, again, another tool in our toolkit to make sure that we are addressing not only the products that are already in the country but preventing illegal products from entering the country.”
As of Sept. 31, the FDA has issued over 1,200 warning letters for online investigations. For manufacturers, the CTP has sent more than 800 warning letters, with more than 750 letters for e-cigarettes. Beginning earlier this year, the FDA also issued the first civil money penalties against manufacturers for violations for illegal e-cigarette sales. He said civil money penalties will remain a part of the CTP’s tools to combat illicit sales.
“We also issued the first six injunctions in coordination with the Department of Justice. I got a lot of flak for that as well about enlisting the Department of Justice. And I will remind folks that the FDA doesn’t have an independent litigation authority. If folks do not comply with the law, we will escalate further, as has been evidenced by these actions, which again are going to be part of our broader portfolio moving forward,” said King. “Everyone is going to be held accountable across the supply chain. We do want to make sure that we address the bad actors in a meaningful way. We also continue to pursue no tobacco sale orders among retailers as well. This has traditionally been issued for underage sales. But again, we’re committed to using the full scope of our authorities granted through Congress.”
King added that education is also a priority for the CTP. The center is ramping up efforts to address misinformation in the continuum of risk for nicotine products. He mentioned that he recently wrote a commentary where he highlighted the importance of opportunities and considerations for addressing misperceptions in nicotine. “There is science that exists in that there are misperceptions around the continuum of risk and also nicotine. And so, we do have opportunities that are present, but we have to follow the data-driven pandemic-based approach,” he explained. “That said, I’m putting my money where my mouth is …. We’re working with the National Institutes of Health for a funding opportunity to get more data on public health communication messaging about the continuum of risk.
“And as noted in that funding announcement, we’re looking for data both for the target population, which is called smokers, but also unintended populations, particularly youth. This is several million dollars on an annual basis, and we look forward to that kickstarting and getting data to inform our work.”
King said the CTP will also continue to gather input from the industry and the public. The CTP is creating a new office within the Office of the Center Director and is looking to hire a new director for Policy and Partnerships. “That posting is public,” he said. “And I’m looking forward to seeing those who have applied and getting someone in that seat to meaningfully oversee the product regulation portfolio across the center, particularly as we get that strategic plan in place.”
During the closing of his address, King said that he continues to be big on communication and stakeholder engagement. He expects to provide the industry with more opportunities for communication with the CTP. “I know that you’ll see in the future an evolution of our messaging. Both through our press releases, our social media and our [overall] messaging to make sure that we are clearer, simpler and more digestible,” he said. “I’ve been a bureaucrat for many years, but that doesn’t mean that I can’t communicate effectively with the general public. I think we can do better. I know we can do better.”
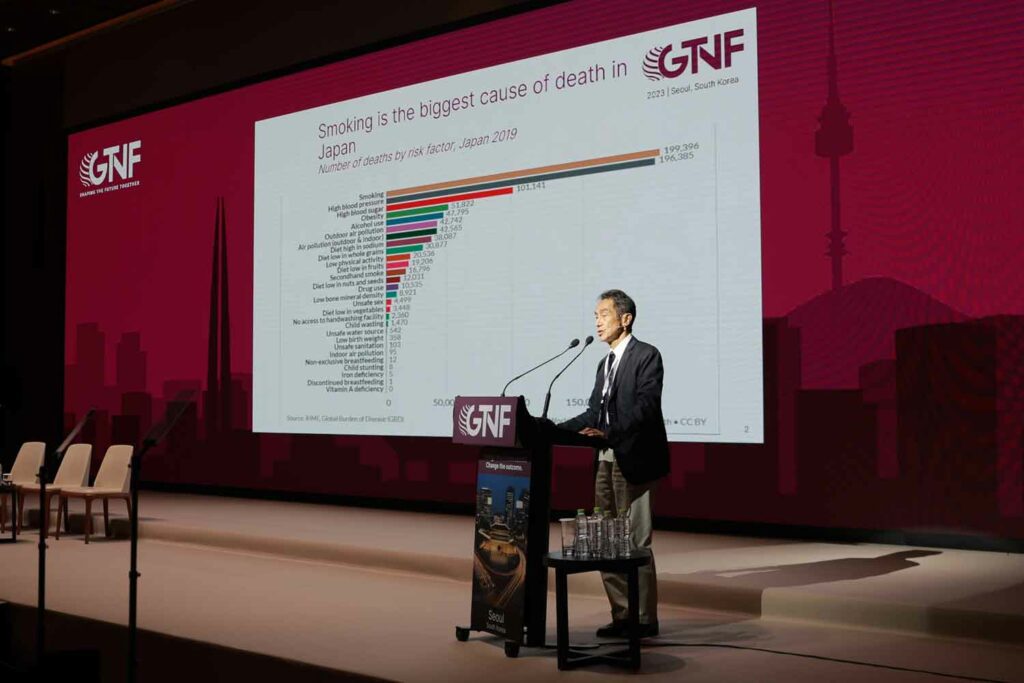
Keynote: Hiroya Kumamaru
Hiroya Kumamaru, vice director at Japan’s AOI Universal Hospital, gave a 15-minute overview of the current status of harm reduction in Japan.
Quoting 2019 figures, Kumamaru said that, as in many other countries, smoking is the biggest cause of death in Japan, though high blood pressure is not far behind and is catching up, probably because of the aging of Japanese society.
On top of this, he put forward the economic argument for reducing smoking, which, he said, while having a positive annual impact on the economy of ¥2.8 trillion ($18.76 billion), mainly through taxation, had a negative impact of ¥4.3 trillion mainly due to loss of labor because of smoking-related diseases, the medical costs associated with smokers and passive smokers, cleanups and fire-related expenses.
Kumamaru told how, about 15 years ago, he had started working in a small clinic in the center of Tokyo, where he became involved in a national smoking cessation program that was based on a three-month-long series of five visits by smokers to doctors. Although a lot of effort was put into the program, and nearly 60 percent of the participants at his clinic went on to complete the five outpatient visits, nine months after the end of the program, nearly half of those who had appeared to have quit started smoking again, a result he described as “disappointing.”
He then compared this with what had happened after the start of sales of heated-tobacco products (HTPs) from 2016. By 2019, almost one-third of male and one quarter of female tobacco consumers were using HTPs, a result that he described as “amazing.” As a result, total cigarette and HTP consumption was heading down while HTP consumption was increasing.
In part, his amazement sprang from the fact that while this was happening in Japan, it appeared not to be happening at the same level elsewhere, and he didn’t know why this was the case. For example, Japan’s smoking rate was decreasing at a faster rate than that of Australia, which had introduced very strict smoking restrictions but banned the sales of HTPs.
Notwithstanding Japan’s success with lowering smoking rates, many people in Japan remained skeptical about HTPs and raised issues about the unintended use of these products: dual usage, initiation, relapse and what is called in Japan the gateway effect of youth initiation. But these turned out not to be significant issues. Kumamaru said about 20 percent of smokers use HTPs and cigarettes, which is not that many. And initiation or re-initiation has been at a very low level, with two years of surveys recording a 2 percent factor in the first year and 1.3 percent in the second. In particular, youth initiation is low, and there has been no increase in initiation among younger people due to the launch of HTPs.
Kumamaru said that interesting data from Italy, Korea and Japan pointed to the fact that consumers of HTPs have better outcomes than smokers in respect of chronic obstructive pulmonary disease and cardiovascular disease while the exposure to carcinogens associated with HTP use is just 3 percent of that of smoking.
He ended his presentation by saying that Japan could look forward to a better future because of HTPs, though it is necessary to keep carrying out surveys and probably starting a long-term clinical study.
