New oral tobacco products have the power to dethrone cigarettes as the leader in nicotine delivery.
Think of combustible cigarettes as dinosaurs. Regulators want them to become extinct. Next-generation oral tobacco products are like the furry little animals scurrying about under the feet of the dinosaurs. These small creatures have the potential to take over when the dinosaurs are gone.
This analogy was presented to attendees of the recent Global Tobacco & Nicotine Forum (GTNF) by health behavior consultant Cheryl Olson while moderating the panel “Oral Tobacco Products — The Road Less Travelled … Until Now?” Olsen said that oral tobacco products are growing in market share and are less-risky alternatives to combustible cigarettes. However, getting consumers to make the switch to any less-risky product is difficult, especially an oral product that doesn’t mimic any of the properties of a combustible cigarette.
Karl Fagerstrom, clinical psychologist at the Smokers Information Center in Sweden, said that a cigarette and an oral product are very different objects, and it can be difficult for smokers to switch from a cigarette to a pouch-style product that is designed to be held between the upper lip and gum. Smokers become addicted to the mouth feel and other properties of the cigarette, not just the nicotine. “The reason for this difficulty is that an individual’s dependence to a drug or whatever is tied to a product. Our brains are not craving an invisible substance but the object that contains the substance,” he explained. “In many circumstances, the individual may not even be aware of the dependence to the substance.”
George Adams, cardiologist for Rex Healthcare at the University of North Carolina Chapel Hill, said that the behavior that goes into the thought process of people who smoke is more personalized than what most people would think. He explained that there are different reasons that people smoke and use these different types of nicotine-delivery systems. He said that a major difficulty in getting smokers to switch are the misconceptions surrounding nicotine in general.
“There’s multiple different products. There are multiple technologies out there. Going from smokeless tobacco products to vaping, gums, pouches, tablets. I mean there’s a whole host of them rather than just the combustible cigarettes that we commonly know,” he said. “As a practicing physician, the thing is that we are not educated on these types of different products. When we think about beneficial effects of choosing products, to help our people who smoke, that causes detrimental effects.”
Doctors are untrusting of the tobacco community, according to Adams. This has led to a disconnect between the science conducted by tobacco companies and health professionals. “I think it’s a detriment to the patients not to have a collaborative effort between the two entities to figure out the best solutions [to help people quit smoking]. We [doctors] are ignorant. We don’t have enough education in terms of the products that are out there and the benefit that they could possibly offer [to our patients],” said Adams. “We know that nicotine is a stimulant; how it affects at the cellular level. And there’s a large group of us—or physicians—that believe that all nicotine products are the same.”
Fagerstrom said that the misconceptions surrounding nicotine are a detriment to public health. He said that many physicians and consumers wrongly believe that nicotine “causes cancer … is causing cardiovascular disease. He believes these misconceptions are the main reason next-generation tobacco products like oral have such difficulties gaining mainstream acceptance.
Adams agreed that many doctors wrongly believe nicotine causes cancer. This makes it difficult for them to recommend any product that contains nicotine, even if a product is 95 percent less harmful than traditional cigarettes. He said the mistrust between the tobacco and health communities runs deep, and doctors do not understand all the different types of nicotine-delivery systems. “It’s a lack of education,” he said.
Mohamadi Sarkar, scientific and regulatory strategist at Altria, said that oral tobacco products have been shown to be safer than combustible cigarettes. He said the vision of oral products is grounded in the foundation of the continuum of risk, which has not only started to gain acceptance in public health but has also been embraced by the U.S. Food and Drug Administration (FDA). “Combustible products are in the extreme end, and noncombustible products, including inhaler and oral products, are on the other end of the spectrum.”
Sarkar showed attendees the results of a study he conducted on harmful and potentially harmful constituents (HPHCs) in oral products compared to traditional cigarettes. “The levels of many of the HPHCs are either not detectable or below levels of quantification. Harmful carcinogens and [other chemicals] are not even detectable,” he explained. “It’s not surprising that these products will completely either eliminate or substantially reduce the exposure to HPHCs, and if this is sustained on a long enough time period, we expect that they would also see a reduction in smoking-related diseases.”
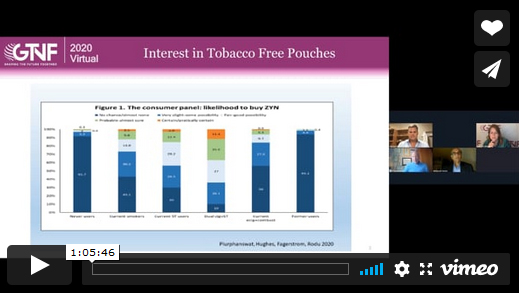
Olsen said she conducted two primarily web-based surveys for another oral tobacco product. She said that her studies found that 43 percent of the never-smokers perceived a high or very high risk of getting a serious illness from using [the oral product] versus 18 percent of smokers not planning to quit,” she said. “And similarly, half of the smokers not planning to quit saw low or no risk versus a quarter of the never-smokers.”
Adams said the solution is education. The FDA needs to allow for better communication about the levels of risk in different types of tobacco products. Nicotine is dangerous and addictive, but if you [are] going to use nicotine, consumers should be able to choose the safest delivery method. “That is a testament to the lack of education that we have across the spectrum. You can’t just group everything together,” he said. “There may be a benefit with certain patient populations to get them off combustible cigarettes … but if you have a closed mind and your shutters are up, you’re never going to have the opportunity to help those patients because you don’t believe in them. It’s a lack of education.”