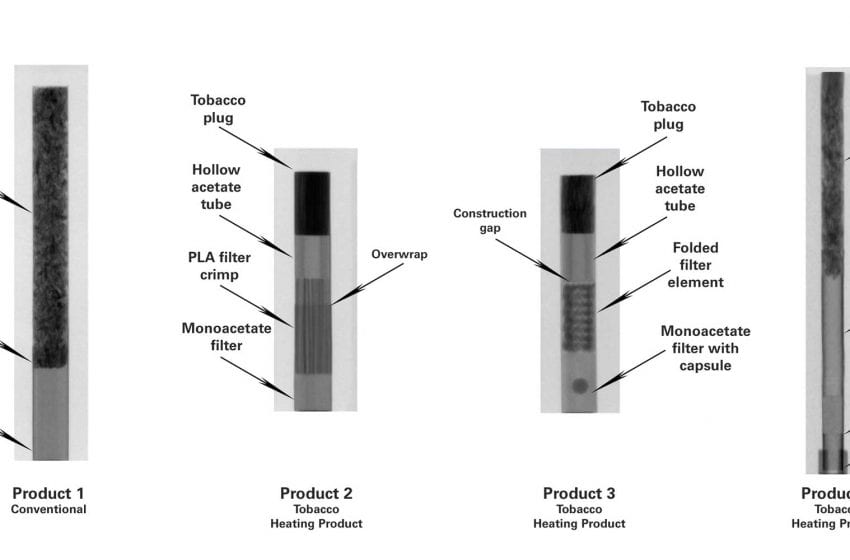
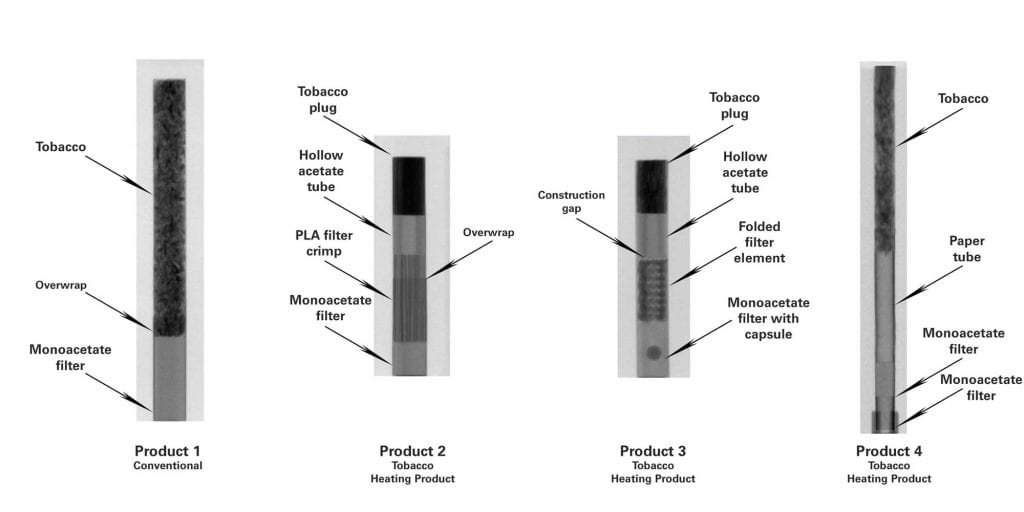
The Fourth Circuit on Monday dismissed an appeal from various vaping groups challenging a compliance deadline for vapor products. The decision states that January directives from the U.S. Food and Drug Administration (FDA) have rendered the appeal moot.
In a per curiam opinion, the appellate judges held that guidance issued by the FDA in January moots the vape groups’ appeal because that guidance supersedes older directives from August 2017 at issue in the appeal and leaves “no possible meaningful relief” that the court could grant, according to law360.com.
“Any ruling by this court as to the procedural or substantive reasonableness of the August 2017 guidance would amount to nothing more than an advisory opinion,” the court said.
The appeal stems from a Maryland district court ruling that ordered the agency to set a May 2020 deadline for premarket tobacco product applications (PMTA) on smokeless tobacco products. The FDA, along with various health and anti-vaping groups, had argued that the January guidance restricting the sale of flavored, cartridge-based vapes rendered moot the vape groups’ appeal.
“Because the enforcement timetable for e-cigarettes set out in the January 2020 guidance is independent of the district court’s order, an order by this court reversing the district court would have no effect on FDA’s enforcement of the statute and regulations against e-cigarette manufacturers,” the agency had previously said.
But the vape groups disagreed, saying the January guidance was enacted without proper notice-and-comment procedures, according to the opinion.
While the court said it can’t offer the vape groups relief in this case, the panel added in a footnote that the groups can challenge the January guidance in a separate action in federal court. The panel also ruled that a Maryland district court did not abuse its discretion in denying cigar industry groups’ motion to intervene, saying those groups did not intervene in a timely manner.
Counsel for the cigar and vape groups and a representative of the FDA did not immediately respond to requests for comment Monday.
Last month, a Maryland federal judge said that in light of the coronavirus pandemic, he would grant a 120-day extension to the May 12 deadline for e-cigarette PMTAs, which have proceeded slowly since the FDA first determined vapes should be regulated like tobacco products. The new deadline is Sept. 9, 2020.
The FDA had previously asked the Fourth Circuit for approval for the lower court to extend the May deadline, saying it would not affect the merits of the appeal brought by the industry groups. The FDA said many of the laboratories and research organizations conducting the clinical trials for the regulatory applications have shut down or otherwise halted in-person testing in light of the COVID-19 pandemic.
Public health groups previously sought to accelerate the FDA’s regulation of vaping products under the Tobacco Control Act, citing vaping-related lung injuries that sickened thousands of people and left nearly 70 dead in 2019. In July 2019, a Maryland district judge effectively allowed the FDA to set the May 2020 deadline, prompting the vape groups to claim the decision was an arbitrary overextension of both the FDA and the court’s authority.
The vape groups had also argued that the May deadline left too little time for manufacturers to file complete applications. Cigar industry groups that filed joint briefs on appeal argued that the district court’s order on deadlines unfairly ensnared cigar and pipe tobacco manufacturers as well.