MedDRA helps evaluators describe the health effects of tobacco products in consistent terms.
By Samina Qureshi
From the time the U.S. Congress passed the landmark Family Smoking Prevention and Tobacco Control Act (FSPTCA) granting the Food and Drug Administration authority over select tobacco products in 2009, the tobacco industry has had to expend vast financial and human resources in efforts to effectively comply.
The FSPTCA in an unprecedented way allows the FDA to implement standards for tobacco products to protect public health. In addition, various statutory pathway applications for new tobacco products must fulfill requirements to record health effects. The applications must have full reports of all investigations to address the health risks of the product. There must also be an established system for maintaining records of health effects. The requirements around recording health effects have to be continued in post-market use of the relevant products.
These requirements have a framework that somewhat resembles FDA requirements for other regulated product industries, such as pharmaceuticals and devices. Although the adverse event recording, reporting and signal detection requirements are much more stringent in these products, the main objective is similar.
The FDA’s usual “safe and effective” standard for evaluating medical products does not apply in the same way to tobacco products. Tobacco products are evaluated based on a public health standard that considers the risks and benefits of the tobacco product to the population as a whole. This “whole” includes users and nonusers. For developing future regulations, the law requires the FDA to apply a public health approach with a focus on the population overall, not just the individual user.
It would be pragmatic to utilize the same standardized international medical terminology that is already being used globally for medicinal products for regulatory communication and evaluation of data pertaining to tobacco products as well.

The terminology used to capture adverse events throughout the drug development lifecycle from clinical trials to investigational new drug applications (IND), new drug applications (NDA) and post-market surveillance is the Medical Dictionary for Regulatory Activities (MedDRA). MedDRA is a clinically validated international medical terminology dictionary. It contains terms that may be used for capturing and recording adverse events experienced by clinical trial subjects as well as in the general population in post-marketing scenarios associated with the regulated product.
The terminology consists of a five-level hierarchy in which the concepts are organized from the most granular lowest level term (LLT) to the broadest system organ class (SOC). The most granular level (LLT) contains multiple terms (over 84,000), which are synonyms or lexical variants of one another but are grouped under a preferred term level (PT) at which level each term is a unique medical concept. Each PT term is further organized under a high level term (HLT) based upon anatomy, pathology, physiology, etiology or function. The HLT are in turn linked to high level group terms (HLGTs). Finally, HLGTs are grouped into System Organ Classes (SOCs). The SOC level is the broadest level and are grouped by etiology, manifestation site or purpose (see Figure 1). There are 27 SOCs in total.
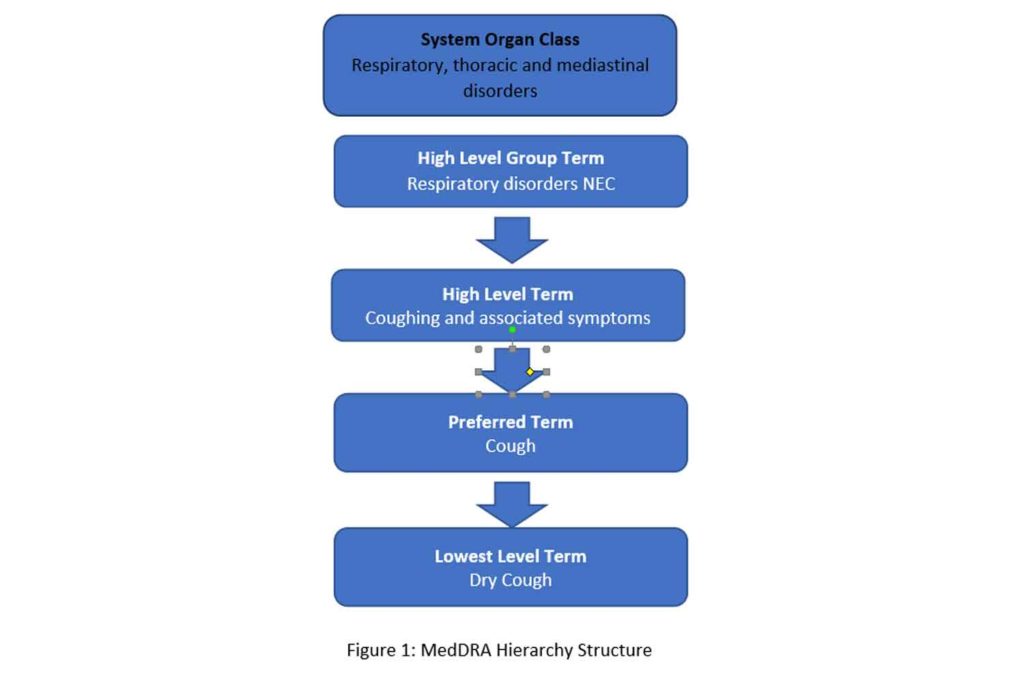

The structure and organization of a concept is very logical in MedDRA and thus supports sophisticated analyses. MedDRA can be used to analyze individual medical events of interest in a database of compiled events or issues involving a system, organ or etiology using its hierarchical structure. MedDRA is a global terminology and is currently mandatory for use in the EU (European Medicines Agency and EU member states) and Japan (MHLW) and encouraged by other global regulators for drug development lifecycle activities including the FDA. MedDRA is listed in the FDA data standards catalog as a terminology to use. Global MedDRA use is facilitated by the fact that it is a multilingual terminology allowing most users to utilize it in their native languages. MedDRA is currently available in 14 languages (Brazilian Portuguese, Chinese, Czech, Dutch, English, French, German, Hungarian, Italian, Japanese, Korean, Portuguese, Russian and Spanish). MedDRA is also a part of the International Council on Harmonization (ICH) e-submission standards.
The functions of maintenance, evolving development and distribution of the terminology to users was tasked to the Maintenance and Support Services Organization (MSSO) by the ICH, who owns the terminology.
MedDRA continuously evolves, and additional concept terms are added with user community input as well as the maintenance organization’s development and proactivity initiatives.
MedDRA is amenable for adaptation and has added terms that address an increasing number of device and product issue terms over the years. There is an opportunity for users to request concept terms with the twice-yearly version releases of the terminology.
Almost all of the ICH regulatory members have implemented MedDRA, including the EU, FDA, MHLW, MHRA, and Health Canada. As global regulators expand their scope of products they regulate, other industries, such as tobacco and cosmetics, have correspondingly started to use MedDRA for capturing adverse health events. It is advantageous for industry users to utilize a terminology in their regulatory applications that is familiar to the regulators who are reviewing the product applications. In addition, it is prudent to leverage the hierarchical nature of MedDRA that is amenable for easy analysis.
MedDRA may be used in all of the statutory pathways for new products in which provisions of the FSPTCA mention recording or reporting adverse health effects related to tobacco products.
An example of potential use of MedDRA in each of the regulatory pathways for tobacco products is shown in the table below, with the regulatory aspect addressed as mentioned in the FSPTCA section.
Statutory Pathways for New Products | Regulatory Aspect | Relevant FSPTCA Section |
Substantial Equivalence Reports | Does not raise different questions of public health | 905 |
Premarket Tobacco Product Application | Appropriate for the protection of public health | 910 |
Modified-Risk Tobacco Product Application | Benefits the health of the population as a whole | 911 |
In addition, the FSPTCA section 909 “Records and Reports on Tobacco Products” states, “to report … information that reasonably suggests that one of its marketed tobacco products may have caused or contributed to a serious unexpected adverse experience associated with the use of the product or any significant increase in the frequency of a serious, expected adverse product experience.”
This particular aspect is best addressed by maintaining a database of adverse health effects reported by consumers on marketed products and may be used to quantify the required events. Pharmaceutical organizations maintain internal safety databases to compile reports of post market adverse events. Many tobacco organizations have adopted this approach and are currently utilizing MedDRA for capturing adverse health effects in their post-market databases. The MedDRA hierarchy is leveraged to perform regular and ad hoc analysis. MedDRA has evolved with the addition of concept terms related specifically to tobacco-related events from the user community.
The hierarchical structure, adaptability to evolve and availability in multiple global languages positions MedDRA as the best terminology for the tobacco industry to increasingly adopt for regulatory applications and for internal systems. MedDRA is also suited for internal assessment of developing consumer products. The product issues SOC is dedicated with concept terms addressing manufacturing, supply, distribution and quality system issues, thus enabling analysis for issues within this realm. It is very useful for analyzing reports of counterfeit products, a common concern. Currently, over 125 countries utilize MedDRA for their regulatory and product development lifecycle activities.








 India closely adheres to the WHO’s abolitionist stance, as evidenced by the country’s 2019 ban on vape products. “My understanding is there is a general denial of harm reduction approaches among governmental stakeholders,” Chowdhery says. “I am not sure, though, how the bans are evaluated or if there is a mechanism for that, though I do keep hearing about smuggling of these goods going up through everyday reports of seizures.
India closely adheres to the WHO’s abolitionist stance, as evidenced by the country’s 2019 ban on vape products. “My understanding is there is a general denial of harm reduction approaches among governmental stakeholders,” Chowdhery says. “I am not sure, though, how the bans are evaluated or if there is a mechanism for that, though I do keep hearing about smuggling of these goods going up through everyday reports of seizures.