Plain packaging has had a measurable impact on smoking rates in Australia, according to Melanie Wakefield, who heads the Center for Behavioral Research at the Cancer Council of Victoria and was also on the advisory group to government on plain packaging implementation.
Data from the National Drug Strategy Household Survey estimated about 11.6 percent of Australian adults smoke daily, down from 12.8 percent in 2016 and more than half the 25 percent who smoked in 1991.
Plain packaging was not the only reform introduced to help bring down the rate, however. Taxes on tobacco were upped by 25 percent in 2010 and then increased by 12.5 percent each year from 2013 to 2020.
Nonetheless, speaking with The Sydney Morning Herald, Wakefield estimates that plain packaging accounted for about a quarter of the total decline in smoking prevalence in three years after plain packaging, leaving Australia with about 100,000 fewer smokers as a result.
Importantly, she says, it has also had an impact on youth smoking rates.
“In the last national survey, only 5 percent of secondary school students had smoked in the last week, and that was down by a third from before plain packaging.”
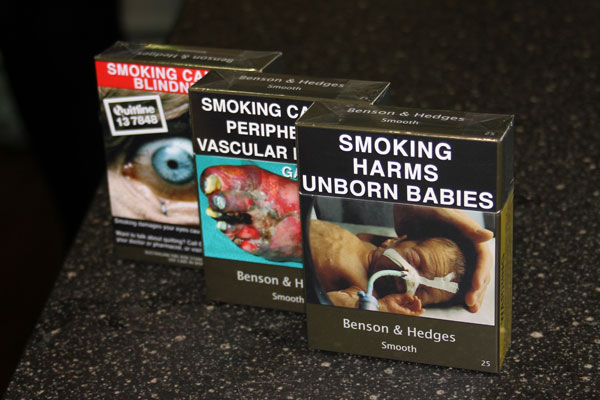
In December 2012, Australia became the first country to require tobacco companies to sell their products in drab olive-brown boxes stripped of branding but featuring large pictures of smoking-related diseases.
Tobacco companies challenged the move in various courts, saying it not only breached trademark laws and intellectual property rights but would also boost black market sales. Libertarians characterized plain packaging as a “nanny state” measure.
Now, 20 countries, including the U.K., Turkey, France, Sweden, Belgium, the Netherlands and Ukraine, have brought in their own versions of plain packaging legislation.



















