It is opportune for the industry to revisit the fundamental purpose of vaping—harm reduction and providing a superior consumer experience.
By Douglas Ming Deng
Pro or against? It is undeniable that disposable e-cigarettes have been the most popular format in the market over the past four years. Despite earlier predictions of their imminent disappearance, recent events like TPE24 and Champs in February suggest that manufacturers and distributors are still committed to showcasing elaborate disposable models. With features such as increased puff capacity, larger screens and vibrant colors, there seems to be no limit to the innovations of the disposables they’re introducing.
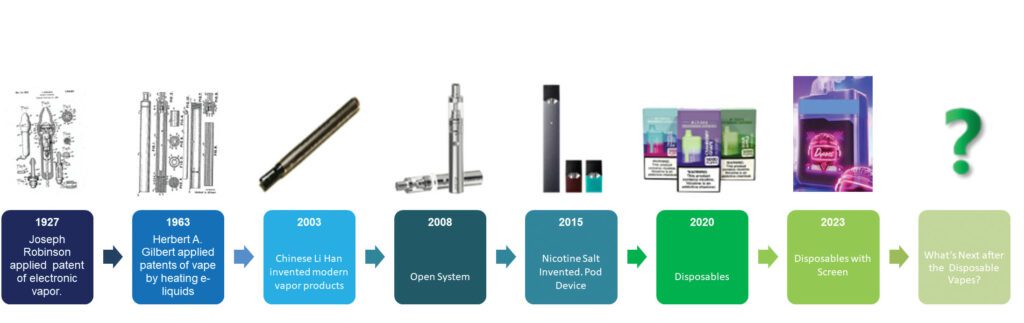
However, changes are on the horizon, especially since the British government announced a ban on disposable e-cigarettes. The ban has sparked growing concerns among industry stakeholders regarding the life cycle of this category. While compliance has always been a central issue, the heightened emphasis on enforcement in the past months has prompted industry-wide apprehension. Yet, for those familiar with the evolution of vapor products, the shift in exterior designs should not be surprising given the industry’s history of transitioning from closed systems to open systems then to pods, pod-mods and now disposables within the past two decades.
At the TPE24 and Champs Trade show, numerous disposable products were exhibited with minimal differentiation among them. Instead of specifying their current preferences for an ideal e-cigarette, both distributors and shop buyers voiced their anticipation for what would become popular six months down the line. To predict the future trends of products, it becomes essential to unravel the reasons behind the success of disposables.
The affordability of disposables lowers the threshold, allowing for a smoother transition for those seeking to switch from combustibles to risk-reduced products (RRPs). Disposables possess clear advantages over device-based systems, particularly in terms of portability, while retaining key features such as noncompatibility of fake cartridges and adjustable coil voltage. In addition, they contribute to relative environmental protection with the increasing volume of liquid filled. However, the downsides of disposables are apparent. Firstly, their disposability sparks significant environmental protection debates, necessitating solutions that incur additional costs and subsequently leading to a general rise in the price of vapor products. Therefore, the next-generation vapor products will be sold at a higher price margin than the current disposables.
Recently, solutions like paper-based bodies with biodegradable plastic components have been introduced to disposable e-cigarettes. In the EU, regulations mandate the rechargeability of batteries in vape products, establishing a reusable device as the minimum standard for the next generation of e-cigarettes. Moreover, the widespread use of disposables among underage individuals has cast a shadow over the entire category since its inception. While recent reports suggest a decline, with some teenagers deeming vaping immature, the issue remains pertinent. If even a single producer persists in designing disposable vapes with toy-like appearances and cotton candy flavors targeting underage kids, the entire industry could face consequences. This negative externality has become more critical than ever, emphasizing the need for the industry to unite and reach a consensus on addressing these public enemies. It is the right time to reconstruct the value of the whole industry and shape a new image of vapor.
Noteworthy Changes
Since the beginning of 2023, the evolution of disposable e-cigarettes has undergone a remarkable surge. For many, the exterior appearance of these products has transformed so swiftly that some manufacturers express concern that their latest models could become outdated before even hitting the market. The size has shifted from compact to large, the weight from light to heavy and the e-liquid tank from small to enormous. The rapid evolution of disposables suggests that the category is approaching the culmination of its development.
One noteworthy change, above all, is the incorporation of screens on these products. While screens have appeared on vapor products before, recent developments significantly differ, particularly from those on open systems. Within just one year, screens have evolved from simple black-and-white displays to color ones then to TFT-LCD, and some brands have now introduced new products with LED touch screens. The on-screen features change from display of battery life and e-liquid contents to fancy animations ranging from alien UFOs shooting off to fireworks blasting, and they seem to emerge one after another. One might question: Are these high-end features really necessary for a disposable vape product priced at $30?
As a scholar closely studying the industry over the past two decades, I strongly believe that the integration of screens on disposable vapes marks a significant breakthrough in vape products and could mean the evolution of vapor toward an advanced step. Interactivity is poised to become the defining characteristic of next-generation e-cigarettes. This interactivity fosters a dialogue between end users and other stakeholders in the industry chain.
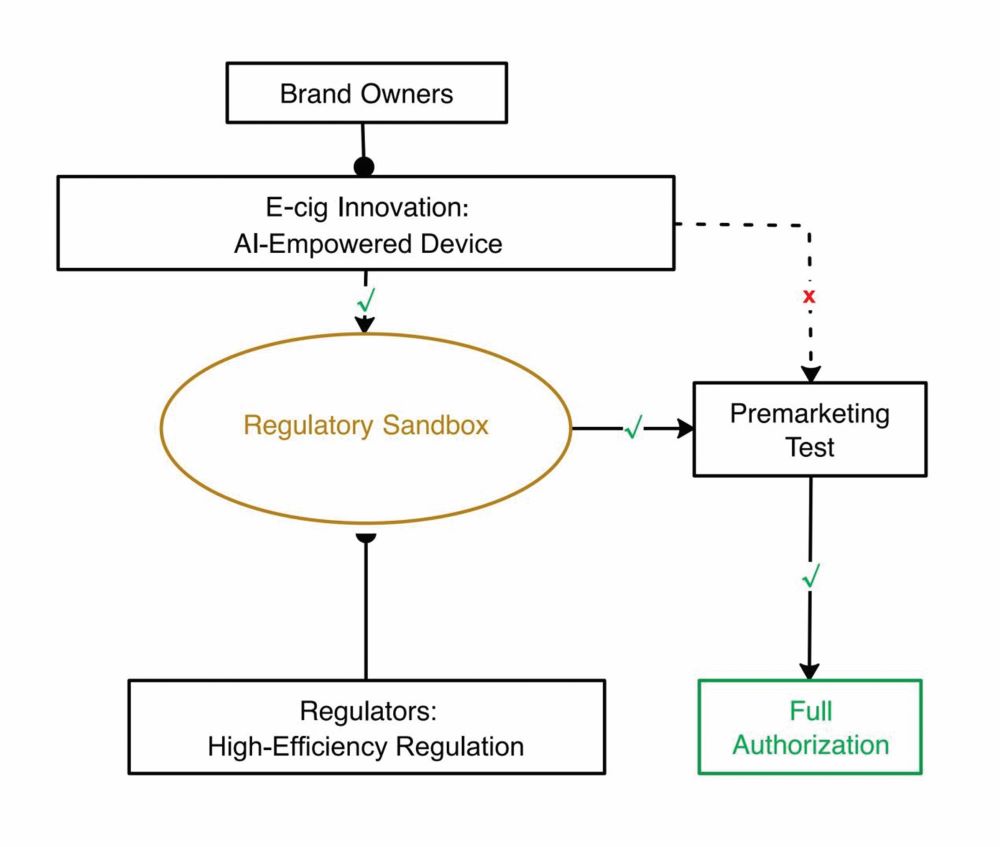
Currently, a major obstacle hindering the expansion of vapor to those who seek RRPs is the lack of communication among manufacturers, sellers, end users and regulators. When end users visit a shop, they often lack knowledge about why they are buying an e-cigarette and what product suits their needs. Shop assistants, with varying levels of expertise, recommend products, and some may lack technical knowledge about flavor differences. Neither end users nor sellers often realize that tobacco harm reduction (THR) is the real selling point of vape products. However, a smart device could facilitate communication between end users, manufacturers and sellers, allowing real smoking experiences to be reported to manufacturers for them to conduct consumer-oriented innovation. During my keynote speech at GTNF 2023 in Seoul, I emphasized the concept of a regulatory sandbox. Such a sandbox would only be viable with the presence of a smart vape device. It would enable vape products to be regulated in a closed loop, fostering innovation by allowing regulators to monitor real-time product testing. Enterprises would receive regulatory feedback promptly, adjusting their research and development accordingly. This approach enhances regulatory efficiency and ultimately builds trust among regulators, enterprises and consumers. Thus, the transition will be achieved from “wait and improve” to “test and innovate.”
In 2022, the introduction of the Lil Aible by KT&G was groundbreaking. This smart device seamlessly integrates the use of heated-tobacco products (HTPs), incorporating both granular and reconstituted tobacco, along with vape technology. The exterior of Lil Aible mirrors the trend observed in disposables today—a robust device featuring a high-definition touch screen. However, the interior features of Lil Aible offer a glimpse into the future of vapor products: the incorporation of an AI function powered by a robust digital CPU. This function not only empowers the e-cigarette to optimize the smoking behavior of each individual user, enabling them to control the total puffs consumed every day, but also enhances harm reduction capabilities. Moreover, the implementation of facial recognition on a smart vape device, when used with due consideration for privacy, can effectively prevent usage by minors. This technology alleviates the burden on regulators for monitoring purposes.
Furthermore, an AI-empowered device would revolutionize the flavoring process. Currently, manual flavoring is often considered an art, with the addition of various flavor chemicals relying on the blender’s personal taste and experience. However, in the era of digital flavoring, the process resembles coding basic substances through chromatographic fingerprints. The flavor can be precisely replicated on an AI smart vape device, a concept known as decoding. It’s crucial to recognize that e-cigarettes possess a natural electronic endowment, making them inherently suited for digital flavoring. In comparison to substances like alcohol or perfume, e-cigarettes have a distinct advantage in executing digital flavoring. This advantage is particularly pronounced in tobacco-flavored e-liquid, where manual methods may fall short of achieving promising results. The inherently possessed electronic capabilities of e-cigarettes may facilitate more straightforward communication between producers and users.
Computer Chips Vs. Potato Chips
Instead of dwelling on the next exterior appearance of e-cigarettes after disposables, it is opportune for the entire industry to revisit the fundamental purpose of vaping—harm reduction and providing a superior consumer experience. Shenzhen, China, renowned as the “Vape Valley,” possesses the capability to spearhead the creation of the next generation of e-cigarettes. During GTNF 2023, we delved into discussions about the future trends of vapor products. Moving forward, professional and technical forums like CORESTA or the Tobacco Science Research Conference serve as valuable platforms to gauge the direction of the next generation of vape products. It is in these forums that the industry can collectively shape the future while staying true to the core principles of harm reduction and delivering an enhanced user experience.
In the years to come, e-cigarettes will embody characteristics of both fast moving consumer goods and more advanced, versatile electronic durable goods. Rather than opting for radical change, the transition from disposables to next-generation devices will be gradual. Like I said during a 2023 industry conference in Shenzhen, involution might only lead to the production of “potato chips” instead of “computer chips.” While potato chips can satiate basic appetites, aiming for the sophistication of computer chips elevates the vape industry to a higher standard. It is crucial for the industry to recognize this potential, as failure to do so may result in being confined to the low-end recycling sector.
In conclusion, the narrative of disposable e-cigarettes mirrors the industry’s dynamic spirit—a story of adaptation, innovation and a relentless pursuit of excellence. The application of screens on disposables might disclose the future of vapor. Standing at the precipice of evolution, the industry is not merely chasing trends but actively shaping a future where harm reduction, end user-centric experiences and technological advancements harmoniously coexist. The tale of disposables is but a chapter in a grand saga, with each exhale marking a step into a future where vaping transcends boundaries and emerges as a beacon of possibilities.