Hiroya Kumamaru, vice director at Japan’s AOI Universal Hospital, gave a 15-minute overview of the current status of harm reduction in Japan.
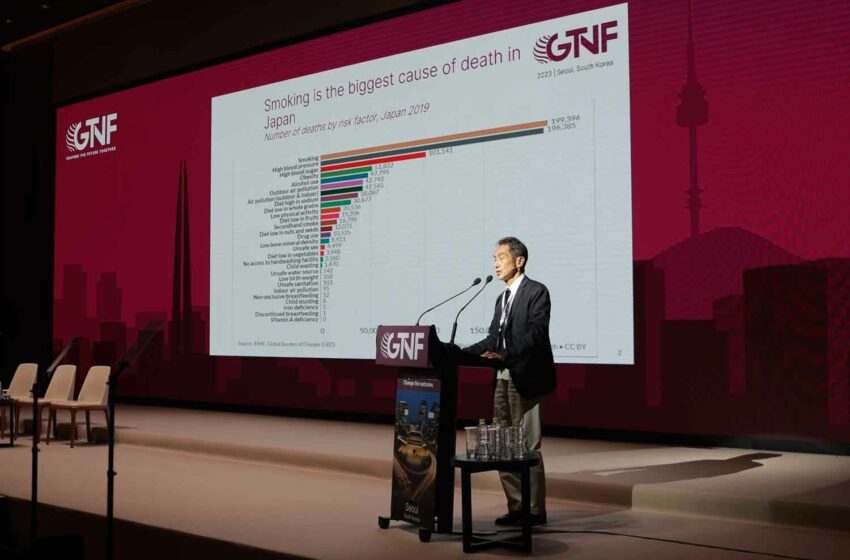
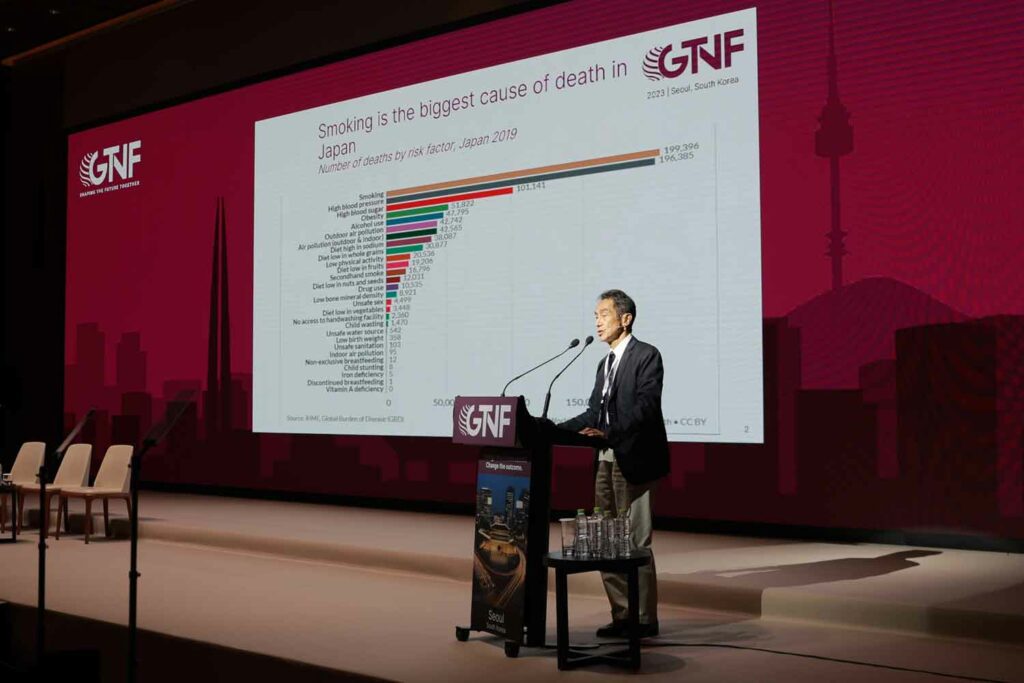
Quoting 2019 figures, Kumamaru said that, as in many other countries, smoking is the biggest cause of death in Japan, though high blood pressure is not far behind and is catching up, probably because of the aging of Japanese society.
On top of this, he put forward the economic argument for reducing smoking, which, he said, while having a positive annual impact on the economy of ¥2.8 trillion ($18.76 billion), mainly through taxation, had a negative impact of ¥4.3 trillion mainly due to loss of labor because of smoking-related diseases, the medical costs associated with smokers and passive smokers, cleanups and fire-related expenses.
Kumamaru told how, about 15 years ago, he had started working in a small clinic in the center of Tokyo, where he became involved in a national smoking cessation program that was based on a three-month-long series of five visits by smokers to doctors. Although a lot of effort was put into the program, and nearly 60 percent of the participants at his clinic went on to complete the five outpatient visits, nine months after the end of the program, nearly half of those who had appeared to have quit started smoking again, a result he described as “disappointing.”
He then compared this with what had happened after the start of sales of heated-tobacco products (HTPs) from 2016. By 2019, almost one-third of male and one quarter of female tobacco consumers were using HTPs, a result that he described as “amazing.” As a result, total cigarette and HTP consumption was heading down while HTP consumption was increasing.
In part, his amazement sprang from the fact that while this was happening in Japan, it appeared not to be happening at the same level elsewhere, and he didn’t know why this was the case. For example, Japan’s smoking rate was decreasing at a faster rate than that of Australia, which had introduced very strict smoking restrictions but banned the sales of HTPs.
Notwithstanding Japan’s success with lowering smoking rates, many people in Japan remained skeptical about HTPs and raised issues about the unintended use of these products: dual usage, initiation, relapse and what is called in Japan the gateway effect of youth initiation. But these turned out not to be significant issues. Kumamaru said about 20 percent of smokers use HTPs and cigarettes, which is not that many. And initiation or re-initiation has been at a very low level, with two years of surveys recording a 2 percent factor in the first year and 1.3 percent in the second. In particular, youth initiation is low, and there has been no increase in initiation among younger people due to the launch of HTPs.
Kumamaru said that interesting data from Italy, Korea and Japan pointed to the fact that consumers of HTPs have better outcomes than smokers in respect of chronic obstructive pulmonary disease and cardiovascular disease while the exposure to carcinogens associated with HTP use is just 3 percent of that of smoking.
He ended his presentation by saying that Japan could look forward to a better future because of HTPs, though it is necessary to keep carrying out surveys and probably starting a long-term clinical study.