Schweitzer-Mauduit International has introduced a new gumming technology to help rolling paper manufacturers protect their brands against counterfeiting.
By Stefanie Rossel
In recent years, cigarette rolling papers have been in high demand; the global roll-your-own tobacco product market, valued at $7.49 billion in 2020, is expected to expand at a compound annual growth rate of 4.2 percent from 2021 to 2028, according to Grand View Research. The increasing preference for handrolled or handmade cigarettes, a cheaper alternative to factory-made cigarettes, has been driving the demand for roll-your-own products. It has been aided by the legalization of cannabis for medical and recreational purposes in a growing number of countries around the world.
As demand for rolling papers has increased, so has counterfeiting of these products. Reports on seizures of significant quantities of fake products, predominantly in the U.S., have repeatedly made the headlines. Rolling paper manufacturers have filed civil actions seeking monetary and punitive damages and injunctive relief from those who traffic in counterfeit goods and who have profited from their sale and distribution. Several leading suppliers of rolling papers have installed dedicated sections on their websites that encourage the reporting of fake products.
While manufacturers fear the financial damage and the harm counterfeit papers cause to their brands’ image, fake products also pose a grave risk to consumers as they are often manufactured using unsafe production practices and unknown and hazardous or toxic ingredients, such as chlorine bleach or petroleum-based adhesives.

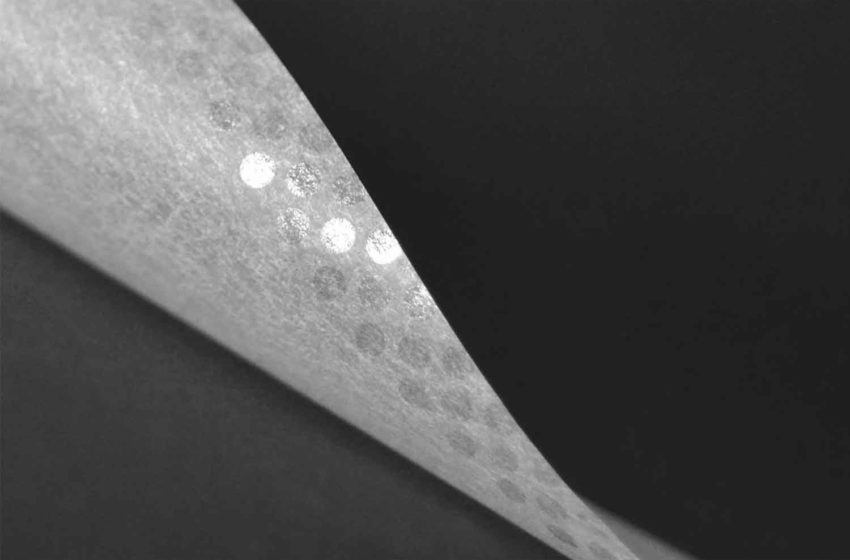
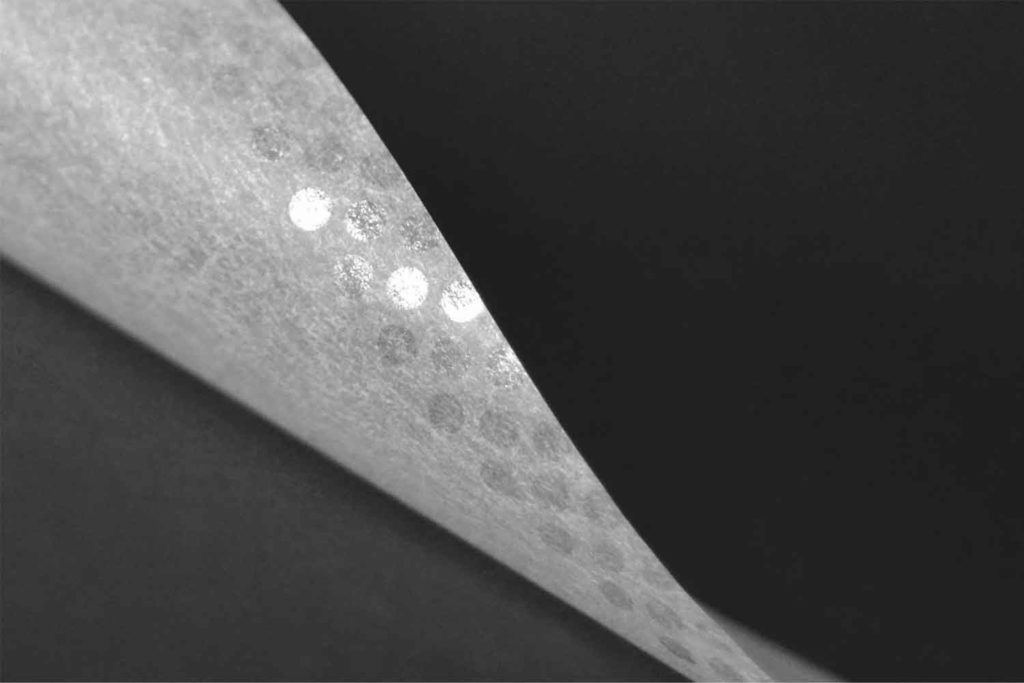
To support its customers in protecting their brands against counterfeiting, Schweitzer-Mauduit International (SWM) has introduced a new generation of gumming technology. “Our new gumming technology is a completely different approach of the existing gumming technology,” explains Pierre Yves Kervennal, product manager for rolling papers at SWM’s engineered papers business unit. “It’s a new space of communication and enhancement of the brand for our customers. The use of natural and colored gum, perfectly in accordance with the legislation, allows us to create patterns, designs and logos inside the gum strip without diminishing the ‘sticking’ quality of the Arabic gum. This opens new opportunities for our clients to differentiate on the market but also communicate with their customers. We called this new service of customization of the gummed band ‘Be Unique.’”
According to Kervennal, tobacco companies are battling counterfeits every day. “It’s not only a financial loss for them but also a reputation hazard,” he says. “And to be fair, the paper industry didn’t bring a lot of solutions to the market over the recent years. Of course, the filigreed papers and the good market practices such as ‘Know Your Customer’ already protect our customers and make it really hard for forgers, but, unfortunately, this is not 100 percent bulletproof. Our clients now have a new weapon at their disposal. Our innovative and patented technology allows to add an additional layer of complexity whilst giving them additional marketing opportunities. In fact, ‘Be unique’ is just an example of the bank note strategy; each time a new bank note is introduced on the market, new technologies are added to the paper to make it safer.”
Driven by Cannabis
In Europe and the Middle East, rolling papers are a historical product of the tobacco industry, Kervennal points out. As such, they face the same kind of issues as any other products from this industry. “It means that major brands can be copied, and with the multiplication of the new distribution channels, the risk of buying counterfeit products is growing for customers. During the Covid crisis and the border closures, we have seen legal markets grow about 10 percent just because the counterfeit products could not come through anymore. In the U.S., this is a very dynamic and trendy market, with some brands already well installed and providing high-quality products. These brands are going to be the ones who will have to fight against counterfeiting—the more dynamic the market is, the more attractive it is for unfair players.”
Responsive to consumer demand, SWM is increasingly focusing on solutions that cater to the growing market for recreational cannabis. “In terms of product design, we develop more and more specific grades and product for cannabis use, such as hemp and unbleached paper,” says Kervennal. Another trend in the roll-your-own market is a desire for differentiation. “We observe an increasing demand from companies creating their own brands and looking for new visual design.”
In the U.S., the RYO papers segment is driven largely by the cannabis market. “Consumers in that market are looking for all-natural products, typically hemp-based products, and brand owners are looking to differentiate their offering through customization,” says Kervennal. “Some additional trend we see in the United States is continuing legalization. Right now, there are 18 states plus the District of Columbia where adult use of cannabis is legal, and that will continue to grow over time. A last trend that we do see is celebrity brand endorsers where brand owners are using celebrities to endorse their brands on social media, which increases demand for roll-your-own papers.”