The case for realistic optimism in the wake of America’s recent election
By Chris Greer
I always admired Donald Rumsfeld’s turn of an opaque phrase. As America’s former secretary of defense memorably explained in a 2002 news briefing on the U.S. invasion of Iraq, crystal ball gazing into the future is nothing if not an embrace of the mist and fog. However, anticipating what may come is not just an amusement, it can be a valuable strategic planning tool during a change of U.S. presidential administrations.
I am often asked to provide my opinion on picks, replacements and likely policy directions. As president of TMA, I cannot provide an opinion. TMA has, for more than a century, striven to be an unbiased convener and place of understanding for those engaged in the complexities of tobacco and nicotine. So why should you continue to read after I just told you that you are not getting an opinion on leadership picks and policies?
I think the perspective offered by a more dispassionate observer is warranted and useful to prepare for January’s changes. There are areas we can be realistically optimistic about, and it is more than an aphorism that what comes next is largely a “known unknown.” That is especially true when we consider the natural starting point of conversations like these: comparing the outgoing administration to what we think will happen during the new one.
A realistic perspective
I will give you one opinion: I believe few people in the tobacco and nicotine industry or harm/risk reduction stakeholder community consider the last four years an uninterrupted halcyon summer. If you do not share my opinion, then we can all at least agree they were eventful and highly consequential. Just naming a few events off the top of my head reveals:
- The early but highly prominent miscommunications on e-cigarette or vaping product use-associated lung injury (EVALI)
- Policy by tweet
- No exemptions for premium cigars
- A ban on most mass market flavored electronic nicotine-delivery system (ENDS) sales
- Uncertain U.S.-China and U.S.-EU trade conditions for leaf and ENDS products
- A global pandemic and its economic and social consequences
- An order by a Maryland federal judge that moved forward, by several years to September 2020, due dates for provisional substantial equivalence and premarket tobacco product application (PMTA) submissions for deemed products
- An order from a Massachusetts federal court directing a rule on cigarette graphic health warnings be promulgated by March 2020
I ask you, is the reality of the last four years really all that different or better than the preceding eight years? Before you answer, consider my list of highlights and where we find ourselves now: farmers struggling, preliminary consumption data indicating no 2020 decline in cigarette consumption, increasing survey evidence showing more people believe any product containing nicotine is no better than a combustible cigarette, a once-in-a-century global pandemic and resulting economic conditions not seen since the Great Depression.
Realistically, despite the overall business-friendly orientation of many Trump policies, what occurred over the last four years was profoundly turbulent and challenging to many aspects of tobacco and nicotine and harm/risk reduction efforts. Not feeling so cheery? Sorry, that was not my intention. But this is the reality we face and, therefore, the starting point for your comparison against what might happen under a Biden administration.

Optimism
Now for the optimism. Provisional submissions for deemed products, including the entire ENDS category, are at the U.S. Food and Drug Administration (FDA). The proverbial elephant left the room. Seems bleak to lead the optimism section with completion of a regulatory submission that only starts an entirely untested process. But it was a millstone around the industry’s neck. And it was an exposed flank that lead to attacks from public health groups against the FDA. What the FDA does with these submissions will be a point of contention in 2021, but it would have been no matter when the submissions ended up being due.
Some of the actions on my list of events from the last four years were the result of trends picked up in the National Youth Tobacco Survey and rising anecdotal evidence of an unwelcome rise in youth using ENDS products. Thankfully, 2020’s survey showed 2 million fewer teenagers falling into the survey’s “user” category than in 2019. Optimists may believe that the tide is turning on youth vape issues. There is a lot to be optimistic about here: Fewer kids taking up vapor products is good for everyone. This trend may absorb a lot of the electricity generated over the last few years. Quieter contemplation may be good for all stakeholders.
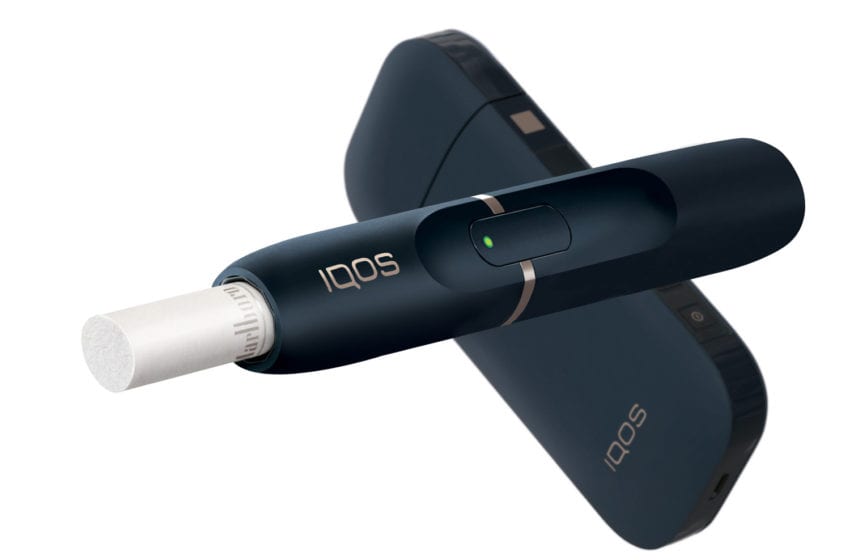
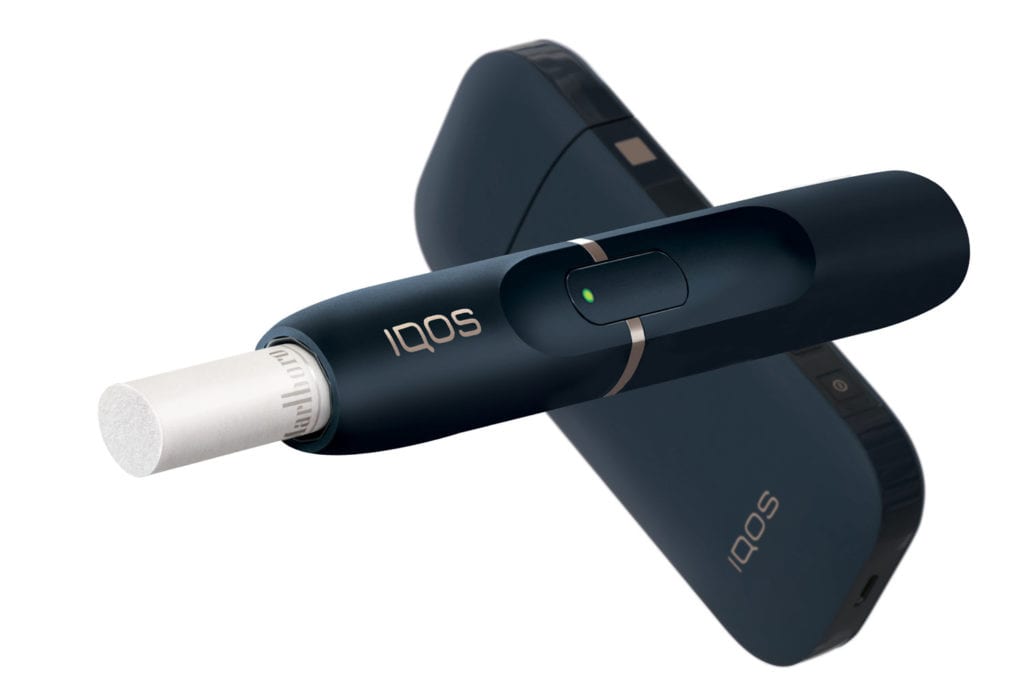
The FDA was also busy making history with several breakthrough authorizations. These include the first modified-risk marketing order for Swedish Match’s General snus and the first modified exposure order for Philip Morris International’s (PMI) IQOS and Marlboro Heat Sticks. This is a big deal. They are not only the first instances of tobacco products that can legally claim users will experience a reduced risk or exposure to smoking-related illness (with exclusive use), but they open the pathway to a number of other products that sit on the lower end of the risk spectrum. Most encouragingly, this was done without hyperbole, based on evidence, following a rigorous but clear(ish) process, in a climate of open hostility toward nicotine-containing products, and it is repeatable for other products pending before the Center for Tobacco Products (CTP).
Lastly, I am writing at the end of 2020, the year best described as a dumpster fire. I feel good that the year is ending, and we have not endured anything else. Honestly, I would not be surprised if we are attacked by the Stay Puft Marshmallow Man or wake up to aliens landing on the National Mall. There is a vaccine or three on the horizon. I am optimistic 2020 will end, and we will all be glad that it never again darkens our doors.
Knowns and unknowns: Knowing the difference
We know things will change. Focus will shift, emphasis will alight on other priorities, political appointments in federal public health agencies will be filled by new people. All of this will occur in the charged atmosphere of a global pandemic, a highly fractured political environment and during an economic crisis.
The biggest unknown is the capacity of the Biden administration to deal with issues not related to the big four: ending the pandemic; addressing the pandemic’s economic fallout; addressing the erosion of faith in the basics of the American political system; and finding the political capital to execute the new administration’s core economic agenda. Each one of these could easily consume all the new administration’s time, energy and goodwill.
It would be reasonable to assume that, regardless of the outcome of Georgia’s January runoff election, the partisan disfunction and the math of Congressional majorities that frustrated much of the Trump legislative agenda after the 2018 mid-terms will persist into the Biden administration. Therefore, a Biden legislative agenda will be difficult to achieve, and the administration will likely be more active on the administrative and regulatory front, especially for noncore issues.
That leaves the tobacco, nicotine and harm reduction agenda in an exposed position, especially given the powers in the vast and unexplored space of product standards. It is conceivable that an active FDA commissioner would implement a more expansive regulatory agenda. However, we must consider the reality of the situation confronting the Biden health policy team.
We are not facing the same level of pressure or attention. Youth ENDS use is trending down. The CTP is quite busy with PMTA submissions. Pathways for modified-risk products, with very robust post-market surveillance, are now functioning and have a pipeline of pending applications. And most curiously, EVALI now languishes in that part of the internet where forgotten news and Paris Hilton reside.
It is a fact that most of the people on the list for responsible positions within the Biden health team have track records in prior offices, or have advanced public positions, that are considered challenging for many harm reductionists and certainly for the industry itself. However, as we have all come to know, facts and truth are different. Truth, while composed in part of facts, is also made of other things like circumstances, possibility, achievability, consequences and priorities. The truth is, we simply do not know what will happen. However, it is equally truthful to point out that the overall instability of the country and an already full plate of the industry’s primary regulator suggests the frying pans remain filled with a lot of uncooked fish.
So, what is my point? Well, we can surmise that tobacco, nicotine and harm reduction will not be a core focus of the Biden administration. Statutory action is unlikely, bouncing the action back to the regulatory sphere. However, the responsible regulatory agencies are also the ones hopefully closing out the pandemic. Some of the key pressure points that emerged during the Trump administration are shrinking, and the CTP has a few items already in its “In” box. What people have done or said in the past is instructive but not determinate for what will occur in the future. Most importantly, and it cannot be understated, one of the core principals of U.S. public health regulation is a basis in evidence and the rule of law.
On this last point, I will veer into opinion once again because my position affords me the ability to compare regulatory structures around the world. The people I meet in responsible federal regulatory positions are thoughtful and dedicated. They always strike me as committed to their mission of going where the science and evidence takes them, upholding the rule of law and taking action in the best interests of the American people—and that includes adult Americans who smoke or are choosing to transition away from smoking using reduced-risk products. Likewise, the industry and large parts of the stakeholder community are similarly dedicated to these principals.
I do look with realistic optimism at the next four years under President Biden. I think we will experience a recovery from the social and economic dislocation caused by Covid-19, and that includes struggling segments of tobacco and nicotine like retailers, small manufacturers, distributors and growers. I trust that our nation can begin to recover and reconcile from the caustic divisiveness that mars our conversations of late. And I hope we can see that fellow Americans are people who may hold different views but are not the enemy incarnate.
The reality of 2021 is much like the reality of 2018–2020: It’s mostly unknown. It is highly charged and divided. It is uncertain and dynamic in ways both positive and negative. It also holds opportunities for the stakeholder community to engage constructively with our regulators as we examine the issue of nicotine and harm reduction. With everyone at the table, dedicated to similar purposes, all stakeholders can advance policies and enact programs that continue to nurture the seeds planted over the last four years.
I invite all stakeholders to use forums like TMA for that purpose. We stand ready to assist the community to turn uncertainty into positive action. Change is unavoidable; the only choice we have is to be shaped by it or take a shot at being the sculptor.