Broughton has unveiled a rebrand that reflects its evolving service offerings to support clients through their whole product life cycle journey. The company states that it is on a mission “to help our clients deliver life-enhancing products to market, by providing the most trusted integrated services in the world.”
Building on years of experience in the pharmaceutical and next generation nicotine products space, Broughton offers its clients fully integrated scientific and regulatory consultancy, combined with comprehensive in-house laboratory services. The launch coincides with the announcement that the business is expanding its services into the rapidly evolving cannabinoids industry.
Moving forward the company will focus on accelerating life-enhancing products to market within strategic markets including pharmaceuticals, nicotine and cannabinoids. Its combined expertise in formulation science, device technology, software applications and aerosol science makes Broughton the ideal strategic outsourcing partner to support client pipeline portfolios of future next generation products, according to the company.
“The launch of the Broughton brand formalizes our rapidly developing position as a full-service solutions provider to the life sciences sector,” said CEO Paul Moran, who founded Broughton Laboratories in 2006. “We will continue our commitment to investing further into global operations delivering scientific and regulatory consultancy combined with comprehensive product development and laboratory services.
“This next phase of our expansion is a natural evolution to grow capacity and capabilities into the broad life sciences sector as technologies improve to target unmet market needs.”
“One exciting aspect of this change is that it facilitates the expansion of our existing pharmaceutical quality and product stability services to support providers of pharmaceutical inhalation products,” said Broughton Chief Scientific Officer Chris Allen. “With expertise in device optimization, human factor studies, navigating complex regulatory pathways for combination products and a track record of quality compliance, our broad expert team can support device development from concept to commercialization.’’
“The expanding team at Broughton hold extensive knowledge of their specialist fields,” said Moran. “This rebrand brings together this expertise with a fresh focus on the journey of our clients’ products to meet an unmet market need. This is an exciting time for the business that will enable us to continue to innovate as we contribute to global health and wellbeing.”
As part of its rebranding, Broughton has created a new website at www.broughton-group.com.









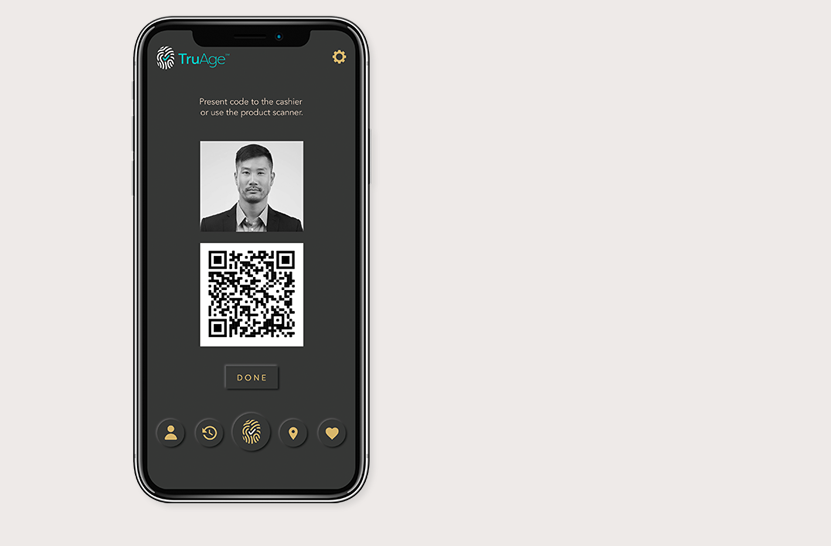
 Altria Group Distribution Co. and Juul Labs have announced their support of TruAge, a new digital solution that enhances current age-verification systems and protects user privacy.
Altria Group Distribution Co. and Juul Labs have announced their support of TruAge, a new digital solution that enhances current age-verification systems and protects user privacy.








 Philip Morris International reported net revenues of $8.12 billion in the third quarter of 2021, up 9.1 percent over those reported in the previous year’s third quarter. Adjusted operating income grew 9.4 percent to $3.55 billion. The company’s adjusted operating income margin was 43.7 percent, compared with 43.6 percent in the third quarter of 2020.
Philip Morris International reported net revenues of $8.12 billion in the third quarter of 2021, up 9.1 percent over those reported in the previous year’s third quarter. Adjusted operating income grew 9.4 percent to $3.55 billion. The company’s adjusted operating income margin was 43.7 percent, compared with 43.6 percent in the third quarter of 2020.
