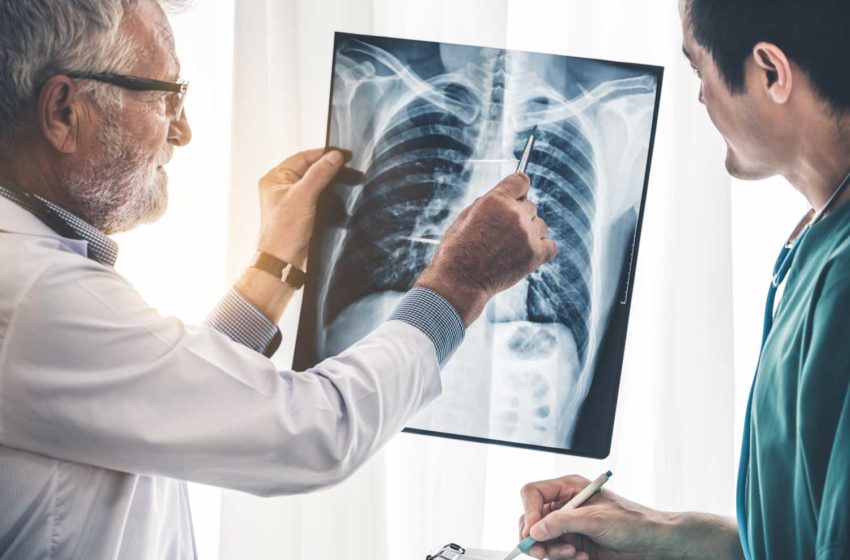
Vaping products are not associated with increased heart attack incidence among people without a history of smoking combustible cigarettes, according to a new study. Published in the American Journal of Preventive Medicine, the paper also concludes that three previous studies claiming a link between e-cigarettes and heart disease wrongly included those who previously smoked cigarettes or were using both vaping products and combustible products. One paper even included participants who had heart attacks before they started vaping.
“Previous researchers confused their own models’ assumptions that these risks were independent with the idea that their analyses validated the presence of independent risks,” the researchers wrote. “There is no reliable evidence that e-cigarette use is associated with ever having had a myocardial infarction among never-smokers.”
Authored by Michael Siegel, a community health sciences professor at Boston University, and University of California, Berkeley, business professor Clayton Critcher, the study analyzed data from 175,546 respondents to the annual National Health Interview Survey from 2014 to 2019.
The researchers found that daily e-cigarette use was associated only with higher heart attack incidence among people who were also currently smoking combustible cigarettes and that there was no evidence for increased risk among vapers who had never smoked combustible cigarettes.
The authors say that the initial study had drawn its conclusions about a perceived cause (vaping) and effect (heart attack) without considering a key variable (smoking).
A 2018 study, also published in the American Journal of Preventive Medicine, claimed that daily vapers increased their odds of heart attack. However, the study only included participants who used both e-cigarettes and combustible cigarettes—none who used e-cigarettes alone.
When challenged by other researchers about their failure to study vapers who had never smoked combustible cigarettes, the authors argued that such a distinction was unnecessary.
In the meantime, two other papers were published based on the original paper’s claims, lending further unwarranted legitimacy to the idea of a link between e-cigarettes and heart attacks, according to an article in Filter.
The second of those two papers was retracted in 2020 for basing its claims on evidence that included heart attacks from before the participants started vaping.