The U.S. Food and Drug Administration properly implemented federal law when it extended certain cigarette rules to other tobacco products and when it assessed fees on cigars and pipe tobacco, the U.S. Court of Appeals for the District of Columbia Circuit ruled on June 20, according to Reuters.
The court unanimously rejected a challenge by the Cigar Association of America, Cigar Rights of America and Premium Cigar Association to aspects of the FDA’s Deeming Rule subjecting cigars and pipe tobacco to the same regulatory framework as cigarettes.
The FDA adopted the Deeming Rule in 2016, identifying a wide range of tobacco products, including cigars and pipe tobacco to be subject to its regulatory authority under the Family Smoking Prevention and Tobacco Control Act of 2009.
The industry groups challenged the rule in the District of Columbia. Some aspects of that challenge have been successful, as when the D.C. Circuit last year overturned a warning label requirement for cigar and pipe tobacco products.
In its recent ruling, however, the appeals court upheld the FDA’s authority to require cigar and pipe tobacco products to undergo premarket review or for manufacturers to provide “substantial equivalence reports” (SE) showing that a product is equivalent to one already on the market before 2007.
The cigar groups had challenged that rule on the grounds that the FDA failed to provide product-specific instructions on how to prepare SE reports and did not consider the cost and benefits of premarket review specifically for each industry or product. They also challenged the user fees on the grounds that the agency did not impose similar fees on other newly deemed products such as e-cigarettes.
Circuit Judge Judith Rogers wrote that it was unnecessary to address whether the law required the FDA to provide the instructions because the agency had said it would give companies 18 months to submit SE reports. She argued that even if the FDA were required to provide instructions, it did not have to do so as part of the rule but could do so later.
Rogers also upheld the agency’s user fees on cigar and pipe tobacco manufacturers and importers, arguing that the Tobacco Control Act specifically gave the FDA authority to impose fees on cigarettes, cigars, snuff, chewing tobacco, pipe tobacco and roll-your-own tobacco.
Anti-smoking activists described the court ruling as “a win for kids, their families and the public health.”
“The D.C. Court of Appeals’ unanimous decision upholding the premarket review provisions of the FDA’s Deeming Rule for cigars will finally force cigar makers to show that their products are substantially equivalent to products that were already on the market before the passage of the landmark Tobacco Control Act,” said Matthew L. Myers, president of the Campaign for Tobacco-Free Kids, in a statement.
“Cigarettes have been subject to FDA regulation since the enactment of the Tobacco Control Act in 2009, but cigar makers were entirely unregulated for seven years until the Deeming Rule subjected them to minimal regulations.”










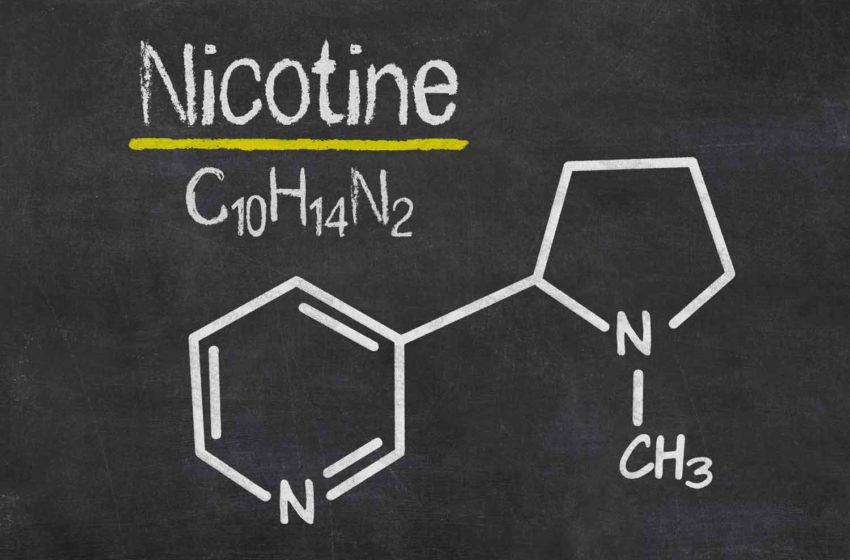
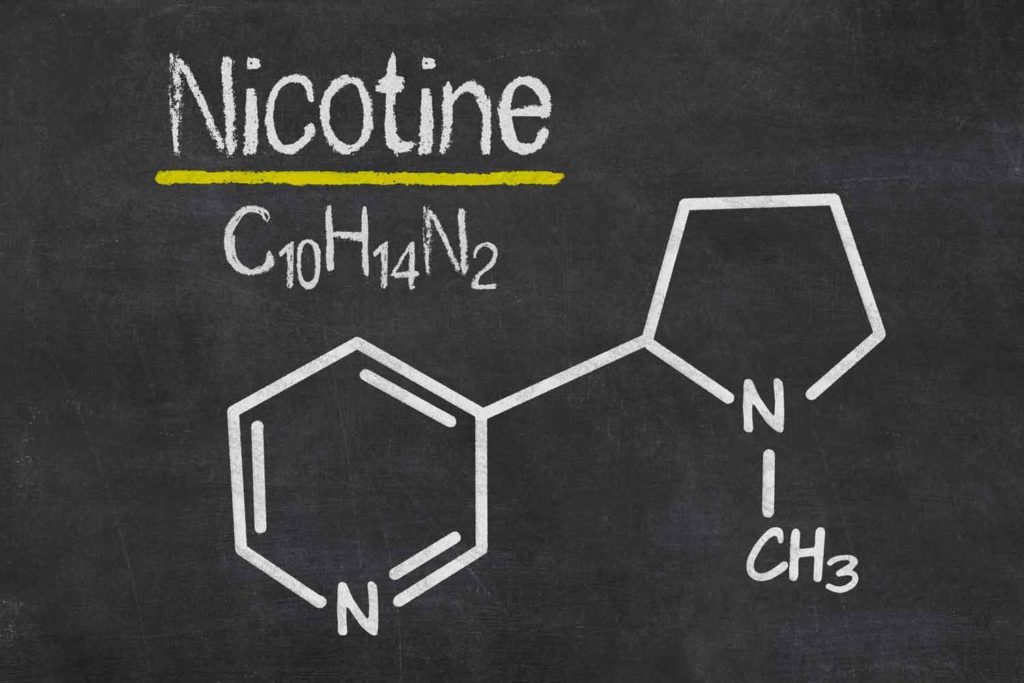



 Kaival Brands Innovations Group is restarting production of its Bidi Pouch ahead of an anticipated September launch.
Kaival Brands Innovations Group is restarting production of its Bidi Pouch ahead of an anticipated September launch.





