With its plastic-free products goal at the forefront, Filtrona’s new filter technology allows for a sustainable RYO option.
By Marissa Dean
When thinking about filters, most people immediately picture pre-rolled cigarettes. They think of discarded butts and microplastics. But those images are changing as the industry evolves and consumers demand more sustainable options. Filtrona is working to fill those needs with its recently debuted trademarked Rip-a-Tip plastic-free filter for the roll-your-own (RYO) market.
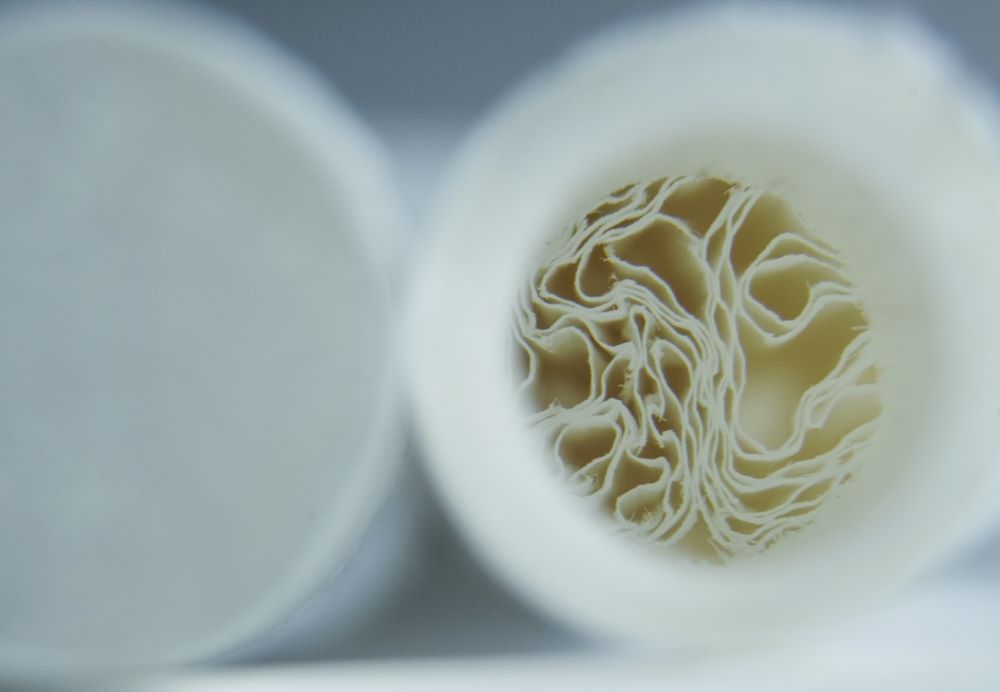
“Made entirely from cellulose, the Rip-a-Tip is designed with convenience and configurability in mind,” says Filtrona CEO Robert Pye. Cellulose is a naturally occurring molecule made up of carbon, hydrogen and oxygen, and it is found in plant cell walls as part of the main structure. Being made completely of cellulose means that the filters will break down entirely upon disposal—removing the potential of microplastics leaching into the environment.
The product is also highly customizable. “It also gives RYO tobacco companies the freedom and options to customize the filter to a preferred diameter, pressure drop and choice of substrate—such as white or unbleached sustainable materials,” says Pye.
“In practical terms, each Rip-a-Tip stick is expertly crafted to hold six individual filter tips measuring 14 mm in tip length. Rip-a-Tip features the EasyRip System, which allows users to rip off the filter tips easily and quickly along the perforated line. This not only delivers an easy and enjoyable user experience but also importantly ensures there is no wastage after the filter tips are ripped off from the outer wrap,” Pye says.
According to Pye, the outer wrap of the product can also be customized with color or print, and the design of the packaging boxes for the filters, which come in flip top, cigarette, push and slide, and side push and slide formats, can be customized.
A Sustainable Future
Like most companies, Filtrona has environmental, social and governance goals that it aims to reach every year. By 2050, Filtrona’s goal is to offer a complete portfolio of plastic-free products.
The Rip-a-Tip supports that goal as “a biodegradable RYO filter solution that meets growing consumer regulatory demand for tobacco products grounded in sustainability,” according to Pye. It “marks an exciting material advancement in the RYO market,” which is expected to reach $45 billion in global value by 2033.
Along with the Rip-a-Tip filters, Filtrona has also launched its trademarked Boreas range of heated-tobacco product (HTP) filters, filling a market need as more consumers switch from traditional combustible cigarettes to HTPs and other reduced-risk products.
The new range includes Boreas SideFlow, a patent-pending filter with a simplified design, and Boreas CoolBridge, a filter that combines Filtrona’s sustainable cooling segment, ECO Bridge, with monoacetate and the company’s patented Fine Wall Acetate Tube to create a balanced retention and cooling mechanism.
“With this new Boreas range,” says Pye, “we are giving customers the option to create a customized, multi-segment filter by combining various types of base rods that are available in Filtrona’s comprehensive collection. As more of our customers move into the rapidly growing HTP market, we are thrilled to be able to support our customers with a dedicated range of HTP filters that bear the hallmark of our innovative and unique designs.
“As consumer demand for HTPs continues to grow, our new Boreas range will enable HTP manufacturers to deliver the next generation of products that deliver the expected level of quality and user experience compared to conventional cigarettes while also meeting regulatory requirements.”

What’s Next?
The industry is constantly changing and evolving as regulations, requirements, health concerns, environmental concerns and consumer desires morph. According to Pye, Filtrona is well positioned to cater to the rapidly changing business environment.
“We have a century of filtration experience and chemistry delivery expertise at Filtrona, coupled with an unparalleled drive for innovation and R&D and world-class global manufacturing capabilities,” Pye says. “In addition, our Scientific Services laboratories in Indonesia offer independent and accredited testing facilities for all our products.
“Our industry knowledge helps companies to catch emerging trends, adapt to changes and build strong brands. We see an exciting future ahead in the industry where Filtrona will play a significant role in driving change and enabling business growth through product innovations, advanced technology and sustainable solutions.”