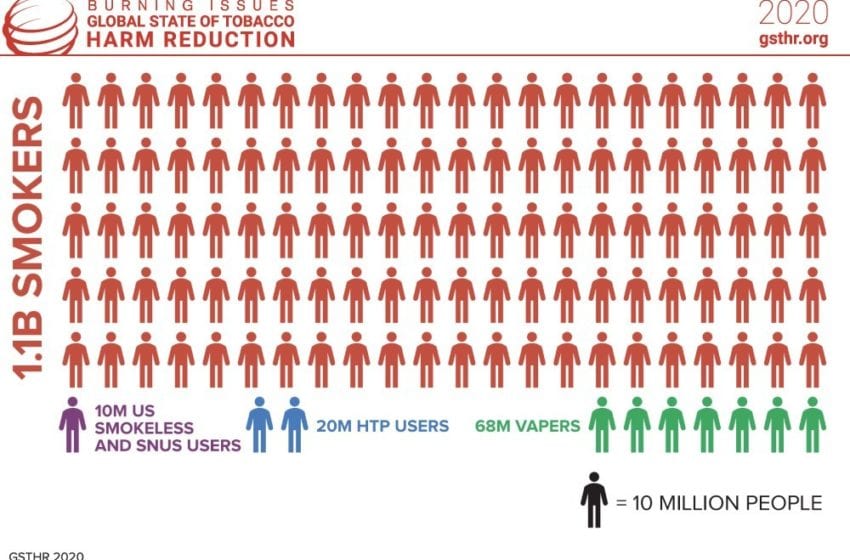
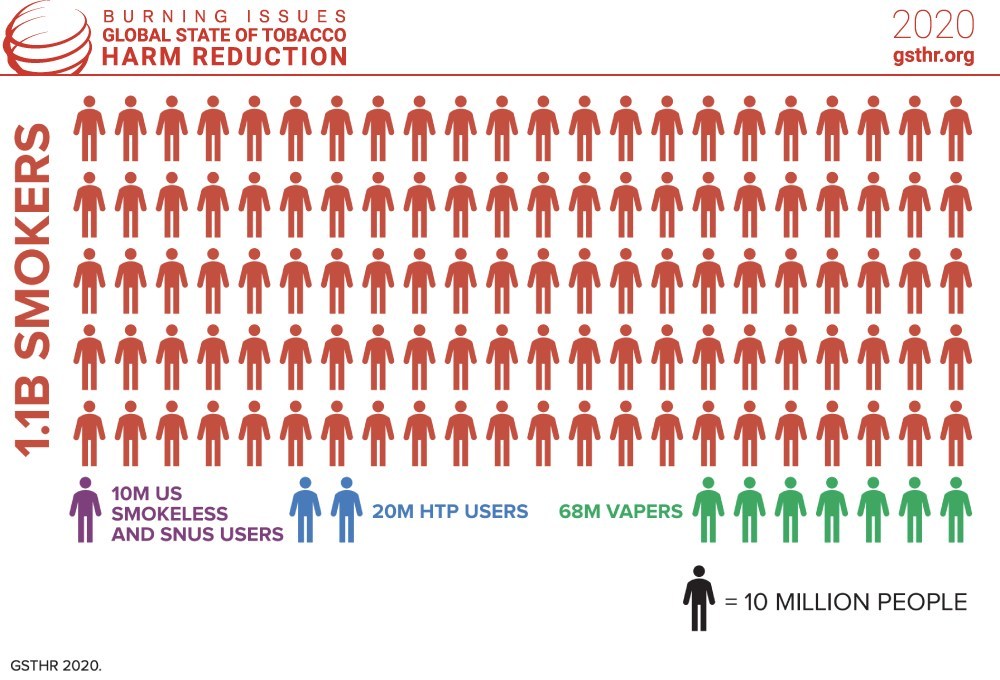
Turkey instructs cigarette makers to use more local leaf
By George Gay
According to the Local Tobacco Inclusion in Tobacco Products law that came into force in October, tobacco manufacturers in Turkey will have to achieve, within four years, a 30 percent inclusion rate of locally produced leaf tobacco within their Turkish market cigarettes.
Currently, the manufacturing industry is estimated to use about 12 percent local leaf, mostly made up of classical oriental tobaccos (COTs) such as Izmir, Samsun and Basma. But while the increases in inclusion rates—17 percent this year, 21 percent in 2022, 25 percent in 2023 and 30 percent in 2023—might at first glance appear to be an opportunity for the COT industry, in reality, the limitations imposed by cost and blend-composition factors will mean that very little, if any, of the increased local demand will be made up of COTs. Additionally, the increased local demand is expected to be met in part by the diversion of tobacco previously earmarked for overseas production centers.
In any case, the requirement to increase local content is expected to see a rise in the inclusion in local cigarettes of sun-cured Virginia tobacco, of which Turkey currently grows about 1,500 tons to 2,000 tons annually but which, under the new requirements, could be expanded to an annual crop of about 15,000 tons. While this type of tobacco does not attract the sorts of prices paid for COTs, manufacturers are likely to meet both blend and cost challenges in including it, partly because an established local illicit cut-rag manufacturing industry uses this type (along with some COTs and imported tobacco) and, without the inconvenience of having to pay taxes, is in a good position to compete for the volumes available.

Meanwhile, producing a bigger sun-cured Virginia crop that is of the required quality and that can be sold for a reasonable price will require dealers and manufacturers to invest in processing and agronomy. The tobacco traditionally grown in the east and southeast of the country was originally cultivated from Virginia seed that, because of the way it was cultivated, adapted to local conditions and produced what became known as a semi-oriental tobacco. The risk of this happening in the future is said to be high unless new seed is introduced and growers are supported in, and compensated for, introducing good agronomic practices. Historically, it is said, growers had little interest in producing quality leaf, preferring instead to concentrate on yields since, for political reasons, they were well compensated for doing so.
Whatever is the extent of the increase in demand that is generated by the new law, it comes at an interesting time because there are supply-side challenges that have been raised by labor issues and the difficulties encountered in introducing mechanization on COT farms—difficulties to do with, on the one hand, the need to overcome farm terrain obstacles and, on the other, the need to finance the necessary investments. Not unreasonably, farmers are not willing to continue growing tobacco unless the returns are, in their eyes, sufficient.
For the time being and the near future, such issues will not be overwhelming because the devaluation of the Turkish lira—down by 40 percent against the U.S. dollar in 2020—will take some of the pressure off grower returns. Additionally, an official inflation rate of 15 percent might allow grower premiums to be paid. But relying on the devaluation of the lira as a long-term strategy would be fraught.

Turning away from local supply issues to those to do with international demand, one factor in the future success or otherwise of Turkish COTs can be examined through the lens of the consumption of American-blend (AB) cigarettes worldwide. Looking at TMA cigarette consumption data, I found 36 countries where, in 2019, AB cigarettes accounted for 50 percent or more of their overall cigarette consumption. Of those 36 countries, 31 countries recorded decreases in the consumption of AB cigarettes between 2015 and 2019, a period that saw falls in the total (all blends) consumption of cigarettes in 28 countries. And looking at the 10 top countries for AB cigarette volume consumption in 2019, the average decline in such consumption between 2015 and 2019 was 10.8 percent compared with 9.6 percent in the case of all types of cigarette consumption in those 10 countries.
On the face of it, this does not look good for the future of Turkish and, indeed, other COTs. But care has to be taken here. Although the above figures give a snapshot of what is going on, it would take a lot of delving to obtain an accurate picture. For one thing, the term “AB cigarette” is used by the tobacco industry fairly loosely, and the inclusion rates of COTs differ widely—down, in fact, to zero—from region to region, country to country and brand to brand within countries.
Given all of the above, it is probably no surprise that Turkey’s estimated volumes of 2020-grown COTs are expected to meet the firm local demand and the less-than-firm overseas demand. The country is thought to have produced about 40,000 tons of Izmir; 4,350 tons of Basma; and 3,500 tons of Samsun along with 10,500 tons of Izmir East (Adiyaman); 3,000 tons of sun-cured Virginia; and 3,000 tons of other types. Overall, Turkey’s crops are thought to be average to above average in quality—slightly better than those of 2019.

But let me digress for a moment. In examining the demand for AB cigarettes, I went looking for those countries with markets with relatively high levels of AB cigarettes to discover whether AB cigarette consumption had declined between 2015 and 2019, but one of the most interesting aspects of the exercise turned out to concern one of the countries where such consumption had risen: Turkey.
According to the TMA figures, Turkey’s American-blend cigarette consumption rose by 0.5 percent between 2015 and 2019. Such an increase will not have set the COT industry alight, but there is something interesting here because Turkey is often in the news for its anti-smoking policies. And yet, it is one of only five countries within my sample of 36 countries where AB cigarette sales rose. In fact, it gets more odd. Turkey’s all blends cigarette consumption between 2015 and 2019 increased by 15.2 percent.
And it gets more odd still. A story on May 31 on the online news site, Ahval, mentioned how the country’s president, Recep Tayyip Erdogan, was a fierce opponent of tobacco smoking and had “long waged anti-smoking campaigns in Turkey.” But despite the fact that the main thrust of the story concerned how Turkey had recorded its highest-ever cigarette consumption in 2019, 119.7 billion, there was no pause to consider whether the anti-smoking strategy might need revisiting, revising and reinvigorating. —G.G.
The author would like to thank Frederick de Cramer, doyen on the Turkish tobacco industry, for his input into the leaf tobacco information and data included in this piece.
Expanding Latakia tobacco production and marketing
Frederick de Cramer, who for many years has been heavily involved in the Turkish oriental tobacco business, has, through his consultancy, Cramer Tobacco, linked with ASTAB in a joint venture (JV) partnership, which aims, among other things, to expand ASTAB’s Latakia tobacco production and marketing.
The JV is using high-quality Izmir leaf and curing it in the traditional Latakia way in barns filled with smoke from burning shrubs that are found only in Turkey and some neighboring countries, a process that gives the tobacco its distinctive aroma.
De Cramer told Tobacco Reporter that this tobacco was highly sought after, especially for inclusion in pipe tobacco blends but also in some other product types.
To satisfy the demand from existing customers, who are mainly based in Scandinavia and the U.S., the JV is planning to increase capacity, in part by investing in new barns. But it is focusing, too, on new customers that have been unable to source Latakia tobacco and have been using mainly artificial flavorings instead.
ASTAB, which was founded in 2007 in Izmir by Haldun Babacan and Selcuk karagozler, was successful in automating the oriental tobacco curing system known as the Vento system and, in doing so, expanded the Izmir crop by more than 10,000 tons in the East Adiyaman area.
De Cramer and ASTAB’s Latakia operations will be the subject of a report in Tobacco Reporter’s February 2021 issue. —G.G.