Navigating successful post-market requirements for ENDS products
By Yvonne Wilding
This month marks the deadline for submitting premarket tobacco applications to the U.S. Food and Drug Administration (FDA). Many organizations have worked diligently to ensure their submissions are as complete and robust as possible. Their goal is to ensure that they are sufficiently complete to allow acceptance for filing and, following substantive review, that the product may be granted a market order by the FDA, allowing it be sold in the United States. By early September, hundreds of PMTAs for electronic nicotine-delivery systems (ENDS) had been submitted to the FDA, and a number are currently undergoing substantive review.
However, in this article I will remind applicants that their obligations do not stop at PMTA approval but persist for the entire life cycle of the product in market—and products can be removed from market potentially more easily than they can be brought to it.
During its PMTA review, the FDA extensively evaluates the provided experimental data to make a risk-benefit assessment of the new product and ascertain its suitability to be designated as “appropriate for the protection of public health” (APPH).
This includes scrutinization of the quality and compliance aspects of the manufacturing processes and review of extensive research information generated on the specific product to allow the FDA to evaluate any potential public health risks associated with the product. The research aspects are compiled by the applicant into different modules of the eTobacco Technical Dossier and include chemistry and manufacturing, toxicology risk assessment and clinical and human experience evidence.
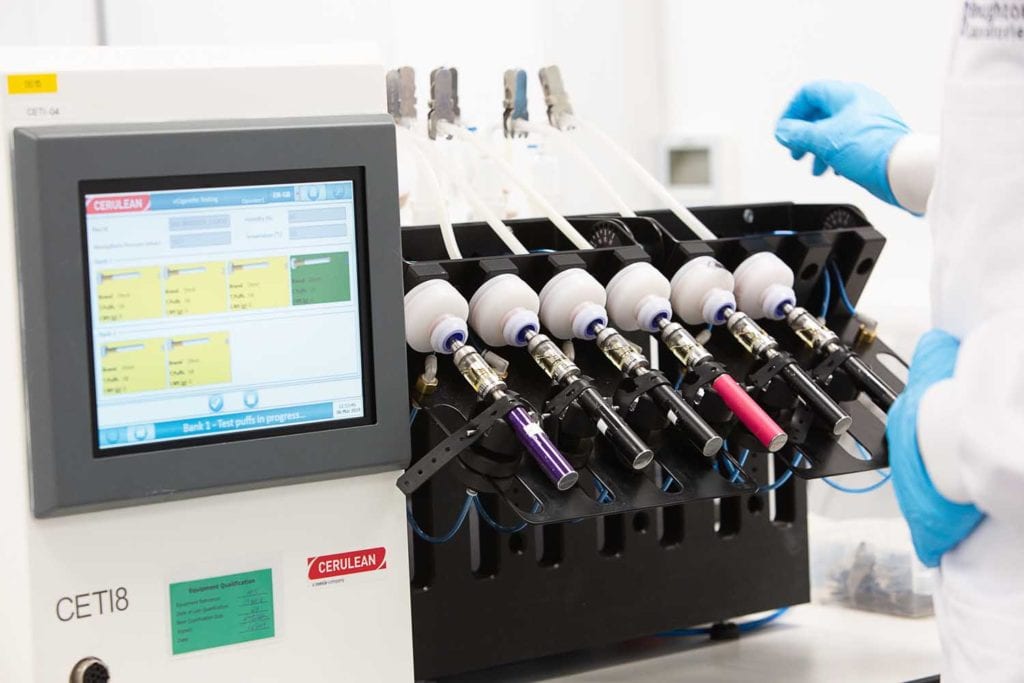
There will be a series of investigations in human volunteers to look at the pharmacokinetics (PK) of the product and its delivery of nicotine compared to comparator products. The PK profile of a product has the potential to affect the abuse potential—i.e., how easily someone may become addicted to nicotine. In addition, there will typically be several human behavior studies to check that the users can operate the device safely and effectively and to assess their preference for this product against competing products. Additionally, there is a significant amount of research done in never-smokers to ascertain how likely they are to start smoking with this product. The numbers of volunteers in the studies are often very large and care is taken to look not only at a representative U.S. population from their demographics but also to incorporate a significant number of young adults in order to be able to make extrapolations to a youth (11–18 years of age) population.
The research data provided to the FDA also includes extensive data on the performance of the device in laboratory-controlled conditions and its delivery of nicotine in each puff under different device settings and different coil components. Stability of the product on storage and extractables and leachables are measured, and quantitative data on the discharge of harmful and potentially harmful constituents (HPHCs)—a list of 33 chemicals of most toxicological concern detailed in the FDA guidelines) is collected. The toxicology and the chemistry data are linked to consumer behavior information and topography data to indicate how a consumer will use the product, enabling the estimation of the likely exposure to potentially hazardous aerosolized constituents and hereby the risk-benefit of this new product can be ascertained.
Assessing the actual product though, is only part of the information required. The product must also be assessed for risk-benefit alongside data from existing relevant tobacco-containing products, much of which is obtained from scientific publications. These comparator products would typically be combustible cigarettes or similarly performing ENDS. It would be wrong to assume that ENDS are without risk, but in a PMTA, the risk relative to other comparator products, e.g., combustible cigarettes, is described. Based on this assessment of these actual and relative risk estimates, the FDA will decide on whether the product is APPH. This approval process can be considered the first step of the journey.
There are several regulatory documents published by the FDA Center for Tobacco Products (CTP). These include Guidance for Industry and Proposed Rule, both of which have sections on post-marketing requirements, although most detail is provided in the Proposed Rule (Federal Register Vol. 84, No. 186, 25 Sep. 2019, Subpart D – Postmarket Requirements section, $1,114.39–$1,114.41).
Having been granted a market order from the FDA, there is an explicit ongoing commitment for each approved SKU to collect and provide information and submit it for regulatory review. Following each review, the FDA will consider whether it is appropriate that the product is maintained in market. Reasons why the FDA may decide to remove a product from market could include any of the following: the product is no longer considered to be APPH; there is inaccurate representation of factual data, the applicant has not set up a system for maintaining records and/or fails to make appropriate records and submit reports.
The post-marketing updates required by the FDA can be put into three main categories: changes to manufacturing processes and controls; changes to the health risks associated with the product; and sales, distribution and marketing information.
Periodic reports need to be submitted to CTP within 60 calendar days of the reporting dates which will be specified in the applicant’s marketing order and must include the following:
Manufacturing and processes
- A description of changes to the manufacturing, facilities or controls during the reporting period
- An explanation of why the changes were made and why these change do not result in the generation of a new tobacco product that is different from the one for which the original order was granted
Health risks
- An inventory of ongoing and completed studies by or on behalf of the applicant that have not previously been reported
- Full reports of information published or known, or which should be reasonably known, to the applicant concerning scientific investigations and literature about the tobacco product that have not been previously reported
- Significant findings from publications not previously reported
- A summary and analysis of all serious and unexpected adverse experiences reported to the applicant or that the applicant is aware of
- A statement of any changes to the overall risk associated with the product and a summary of the health risks, including the nature and frequency of the adverse experience and potential risk factors
Sales, marketing and distribution
A summary of sales and distribution of the tobacco product for the reporting period to include:
- Total U.S. sales and demographic characteristics of purchaser
- Specimens of labelling and detail of any changes
- Full-color copies of all advertising material used with dates of dissemination
- A description of advertising and marketing plans
- Actions taken to restrict youth access and limit youth exposure to labelling, advertising or promotion
- Use of social media
- Use of partners, influencers or bloggers
- An assessment of the impressions left by advertising and audience demographics
In addition to the above categories, there is also a requirement to provide any additional information specified or any additional requests under the terms of the marketing order and an overall assessment of how the product continues to be APPH.

As well as the requirement for regular submission of post-marketing reports, if there are any serious and unexpected adverse events reported to the applicant, these must be reported to CTP’s Office of Science via the Health and Human Services Safety Reporting Portal within 15 days of the applicant having received the report.
There are strong concerns among the public, health policy makers and government that the potential health benefits of switching smokers from combustible cigarettes to a less harmful way of accessing nicotine may be dramatically offset by young nonsmokers being attracted to vapor devices and through use of these devices drive an increase in nicotine addiction in youth. Smoking volumes of combustible cigarettes have been falling for many years across the world, which will ultimately lead to significant improvements in health, although the evidence will take several years to become apparent. Understandably, there is much enthusiasm that the scourge of tobacco smoking-related diseases may be, if not eliminated, significantly reduced in future generations.
It is in the interest of consumers who may wish to move to a safer form of nicotine intake that PMTA applicants are fully compliant with the post-marketing requirements and continue to work with the FDA and lobbyists to contribute to and maintain an appropriate balance of risk reduction and health benefit realization. If these products, stated to be APPH, are no longer available to consumers, then the consumers’ opportunity to potentially improve their long-term health outlook is diminished.
Many of the larger companies manufacturing ENDS products may have specific regulatory compliance staff and sales and marketing departments that are fully trained, equipped and resourced to deal with the FDA requirements, but for the smaller organizations, without such infrastructure, this resource requirement, skill set, potential complexity and the associated costs, particularly if the organization has a large number of SKUs, can be particularly onerous.
The cost and resource requirements of maintaining established products in market has been recognized in the pharmaceutical industry for a very long time. As pharma companies typically prefer to invest in novel medicine development or in the enhancement of existing medicines to provide patent life extensions, some older, established products are still very successful and so the maintenance of the post-marketing requirements of these products from a regulatory and pharmacovigilance perspective is often outsourced to specialist contract research organizations (CROs) who collect and report this data on behalf of the pharma companies. These CROs can provide the necessary resources, expertise and experience to make this a cost-effective solution.
It will be interesting to see how many ENDS products are successful in receiving a positive market order decision from the FDA only to be subsequently removed from the market as they fail to satisfy their post-market approval requirements, causing these products to be potentially as ephemeral as the aerosols they disperse.