Rules to reduce nicotine in cigarettes are back on the agenda.
By Clive Bates
On June 21, the United States Federal government announced its intention to develop a rule requiring deeply reduced nicotine levels in cigarettes on sale in the United States. This idea has been in circulation since first proposed in 1994 and was given a new lease of life in the FDA comprehensive plan in 2017. In late 2021, New Zealand adopted this policy and has committed to introducing legislation this year. The zombie policy is back, and it walks among us. Despite the warped logic of taking out the relatively benign nicotine and leaving the very harmful tar, despite the obvious practical problems of mounting a de facto cigarette prohibition at this scale, and despite the weird ethics of encouraging people to switch from one smoking product to another smoking product, this idea just will not die. How should we consider this proposal from a tobacco market transformation perspective?
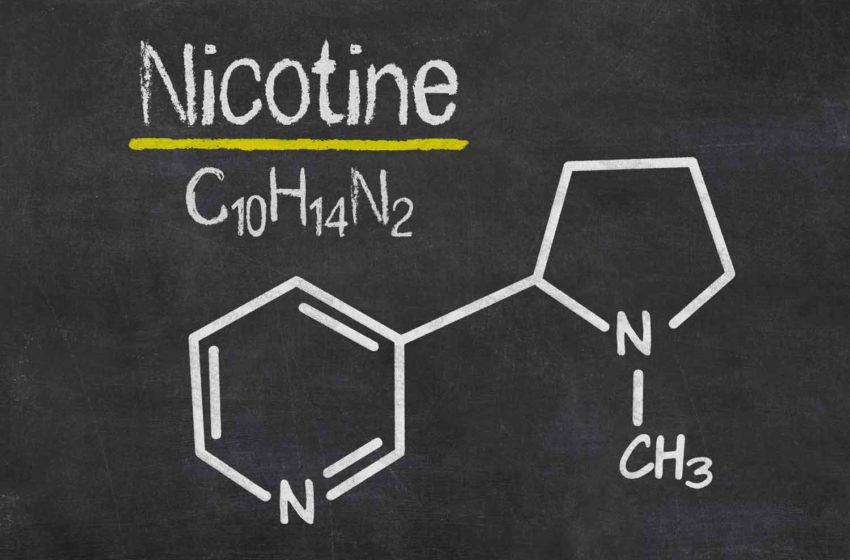
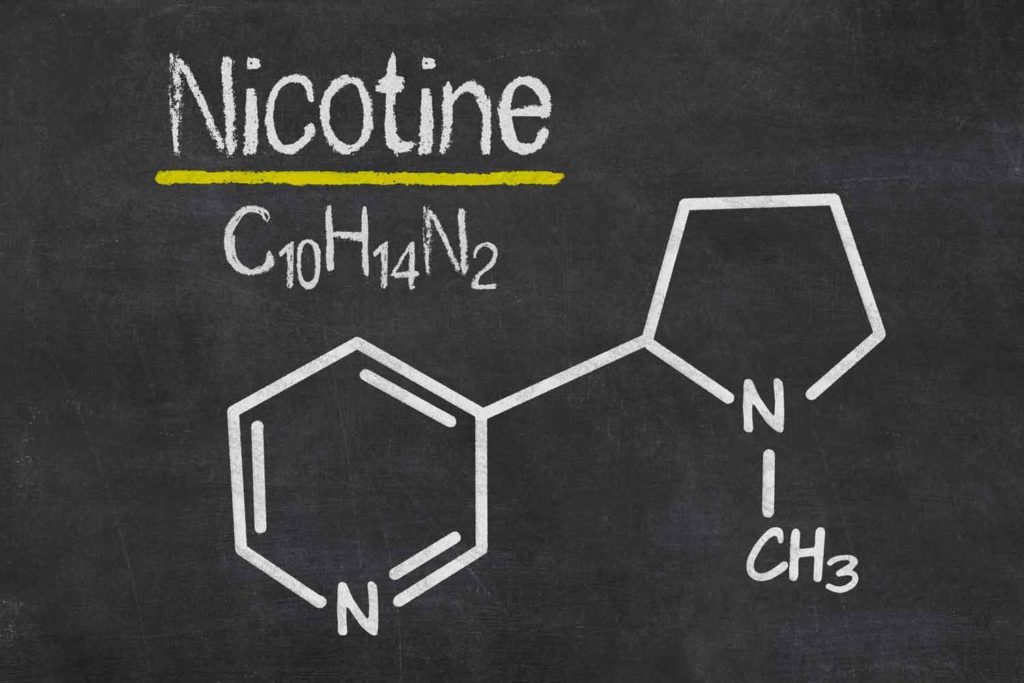
First, let us consider how this would work in practice. Regulators would introduce a rule requiring that cigarettes must have a very low nicotine level in the tobacco, say 0.4 mg per gram of tobacco compared to typically 16mg per gram—a 40-fold reduction. The level would be so low that nicotine could not play a meaningful role in the smoking experience, and “compensation” to obtain a satisfactory nicotine dose by puffing harder would not be possible. For unexplained reasons, regulators and researchers think that people would continue to buy and use these products, but smoke less and not become addicted to them. That forms the basis of the public health rationale for such a rule. But this is absurd.
Second, the science is weak. Given that the main reason people smoke is to experience the effects of the stimulant nicotine, using low-nicotine cigarettes is among the least likely of all the possible responses to such a rule. After trying such products once, few smokers be willing to hand over hard-earned cash at the corner store for cigarettes with no noticeable nicotine. Yet nearly all the science to support rulemaking assumes that smokers will use these products and then finds out what happens if they do. But trials are not markets. In a trial, the subjects are volunteers and agree to participate. They are paid or incentivised to stick with the trial protocol and are usually provided with free cigarettes. This is nothing like real life.
Third, what matters in real life is how users and suppliers respond to the disruption. A low-nicotine rule would eliminate the lawful supply of nicotine cigarettes. But it does not make them disappear from the market. It changes who supplies them, how, and at what price, creating incentives to develop a black market. The established producers would not remain passive either. They will find other lawful ways to sell nicotine-bearing smokes, for example, by selling cigars or hand-rolling tobacco or promoting workarounds such as personal cigarette-making machines. Consumers who use nicotine cigarettes will not automatically become abstinent or lose their interest in nicotine just because the FDA introduces a rule. Maybe they switch to black market cigarettes, cigars, or vaping. Nothing remains constant. A new rule disrupts the market, but it does not mean smokers will automatically do what regulators hope they will. The question is, what would they do, and can this be influenced?
Fourth, regulators must shape the behavioural and market response to the rule. Merely implementing a low nicotine rule does not determine its public health impact. The pattern of behavioural responses to the rule will determine the overall effect. “Good pathways” like switching from smoking to vaping will compete with “bad pathways” like switching to black market products. Confidence in good behavioural responses to a rule should be a precondition for its introduction. Suppose we hope a reduced nicotine rule would prompt a migration of smokers to smoke-free alternatives. Three conditions would need to be met to incentivise migration from smoking to vaping. (1) the smoke-free products must be affordable, attracting zero or minimal tax. (2) the products would need to appeal to smokers by offering a diverse selection of flavours, good nicotine delivery, and a range of devices to suit all needs. (3) consumers must be aware of the benefits of switching, notably the dramatically reduced health risk compared to smoking. Sadly, there is no sign that the U.S. tobacco control community is working towards these meeting these preconditions, though there is a little more policy coherence in New Zealand.
Fifth, there is a paradox at the heart of this proposal. A reduced nicotine rule needs good pathways to the low-risk options discussed above to make it viable. But when those preconditions are met, a low nicotine rule loses most of its purpose. Meeting the preconditions for a rule is a more useful policy than the rule itself. When those conditions are met, everyone who wants to quit smoking by switching can make an informed choice and just do it. What is the case for coercing the smoking holdouts? Surely, the state’s role should be to get in their corner with help and encouragement, not a big regulatory stick. And what if some people are fully aware of the risks, fully understand their options to switch to high-quality, low-risk alternatives, but still don’t want to switch? Should they be made to? And anyway, would anyone in public health want smokers to switch to a low-nicotine cigarette when they could be encouraged to vape? Imagine if a manufacturer brought out a vaping or heated tobacco product with the same nicotine delivery and toxicity profile as a low-nicotine cigarette? No one would take such a product seriously, let alone propose it as a public health intervention.
Sixth, but what about youth? One of the big ideas that underpin this proposal is that non-addictive cigarettes would stop teenagers from falling into the trap of addiction and lifelong smoking and harm. This is now a much weaker argument than in 1994 when the reduced nicotine cigarette idea was conceived. In 1994, 12th grade past-30-day smoking prevalence was 31 percent. In 2021, it was down to 4 percent, according to the University of Michigan Monitoring the Future survey. The current trend in youth smoking is sharply downwards from a low base. Teenage vaping is likely doing the job that reduced nicotine cigarettes were once supposed to do – eliminate teen smoking. The other argument is that no evidence exists that this de facto cigarette prohibition would work. Consider the case of youth marijuana use. The Monitoring the Future survey shows that 12th grade past-30-day prevalence has been steady at an average of around 21 percent since 1994. But throughout most of this period, marijuana has been completely prohibited. The point is that banning something does not make it disappear or mean that teenagers cannot access it. It is more likely to mean they participate in illicit supply.
Seventh, the politics will be very fraught. Though the legal base for such a rule would be Section 907 of the Tobacco Control Act, it would be a mistake to believe this could be done as a narrow technocratic rule-making exercise. Such a measure would impact a wide range of stakeholders far beyond the FDA’s routine contacts. U.S. cigarette sales are about $80 billion annually, and every part of that supply chain, from farmers to convenience store retailers, would be affected. There would be a substantial hit on the cigarette tax and master settlement receipts to State budgets. Law enforcement would be drawn in, with the potential for some of the adverse impacts we have seen in the War on Drugs. Then there is the question of identity politics. Surely, tobacco control activists have noticed that even sensible public health responses to the Covid-19 pandemic became weaponised into polarising identity politics. How would the Federal government intervening in the personal behaviour of 34 million American smokers play out in the febrile and divisive political climate that has developed in the U.S. since 2016? Which political leader will take a hard look at this measure and conclude, “yes, that is just what I need to win the next election”?
In my view, measures like a low-nicotine rule are fantasy “policy masterstrokes”, in which a massive problem is solved with the swish of the regulator’s pen. I doubt this measure will ever work in real life. It will be mired in a practical, legal and political quagmire until it is no longer relevant. In the meantime, it is a distraction from the more useful, feasible and respectful regulatory business of setting up risk-proportionate regulation, fair taxation, and honest risk communication about smoke-free nicotine products. Migration of the market for consumer nicotine from smoking to smoke-free is the practical and viable way to make cigarettes obsolete and end the epidemic of smoking-related disease. A policy that relies on the consent of users rather than prohibition and coercion is far more likely to succeed.