 The U.S. Food and Drug Administration has rescinded another marketing denial order (MDO), placing Fumizer’s flavored vapor products back under review, reports Filter. Fumizer received its MDO in September.
The U.S. Food and Drug Administration has rescinded another marketing denial order (MDO), placing Fumizer’s flavored vapor products back under review, reports Filter. Fumizer received its MDO in September.
This recission comes just weeks after the agency withdrew an MDO issued to Turning Point Brands (TPB).
In a letter to Fumizer’s, the FDA stated that “upon further review of the administrative record, FDA found relevant information that was not adequately assessed previously.”
“Specifically,” the letter states, Fumizer’s “application did contain randomized controlled trials comparing tobacco flavored ENDS [electronic nicotine-delivery systems] to flavored ENDS as well as several cross-sectional surveys evaluating patterns of use, likelihood of use and perceptions in current smokers, current ENDS users, former tobacco users and never users, which require further review.”
The FDA has indicated that it “does not intend to initiate an enforcement action” on Fumizer’s flavored vapor products returning to the market during the new review.
Many MDO recipients have complained that the FDA has been “shifting its goal posts,” during the review process, demanding certain studies that it did not appear to require before the PMTAs were filed.
According to industry insiders, the most recent MDO recission demonstrates that TPB’s successful petition for review and motion for a stay wasn’t a one-off, resulting from the legal jurisdiction it was filed in.
“A rescission in California for Fumizer is evidence of the systemic failure of the agency to ‘adequately assess’ the science and data of a wide range of small- and mid-sized applicants while giving all of their time and attention to the large companies like Juul and Reynolds,” a source told Filter
Multiple companies have challenges their MDOs. Triton, Bidi and Gripum recently received some temporary form of stay, and My Vape Order has demanded a recission due to the fact its PMTA includes some of the same data and studies that also appears in TPB’s applications.

















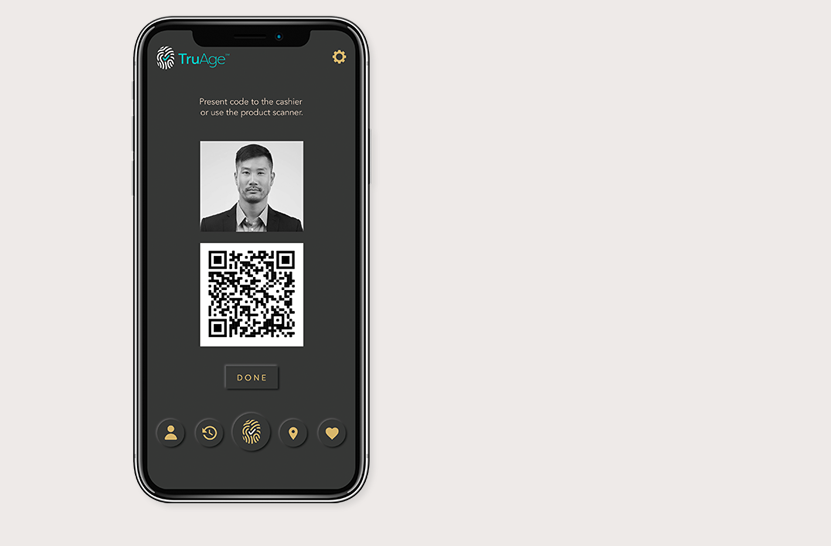
 Altria Group Distribution Co. and Juul Labs have announced their support of TruAge, a new digital solution that enhances current age-verification systems and protects user privacy.
Altria Group Distribution Co. and Juul Labs have announced their support of TruAge, a new digital solution that enhances current age-verification systems and protects user privacy.
