Restrictions on flavors in vaping products would be a drastic setback in the battle to reduce the 48,000 Canadian lives lost every year to smoking, according to a new independent study released on Jan. 11.
The review, covering more than 340 articles of evidence on e-cigarette flavorings, concludes that they are “inextricably linked” to smoking cessation and should be made more accessible and affordable to adults trying to quit.
“Well-regulated use of flavors can and should be considered as a valuable tool to help prevent disease and save the lives of adult smokers who cannot or will not quit by themselves or with other approved methods,” says report author Konstantinos Farsalinos, a cardiologist with a career devoted to tobacco harm reduction.
Farsalinos released his review at a webinar on Dec. 15, 2021.
The report, which examines the science, consumer insights, risks and regulatory considerations related to e-cigarettes, comes as Health Canada seeks to implement a ban on flavored vapes.
“We’re at a crossroads where policymakers are about to turn away from the evidence showing flavors help smokers transition to products that carry only a fraction of the risk of combustible cigarettes, thereby preventing disease and saving lives,” said Farsalinos.
“If bans were allowed, it would ultimately drive consumers to tampering, illicitly traded products, towards the black market, or back to traditional cigarettes.”
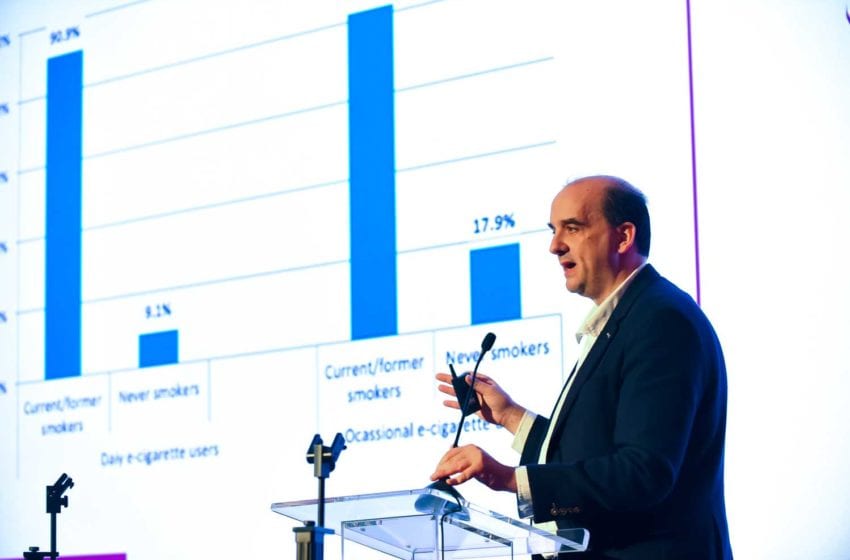
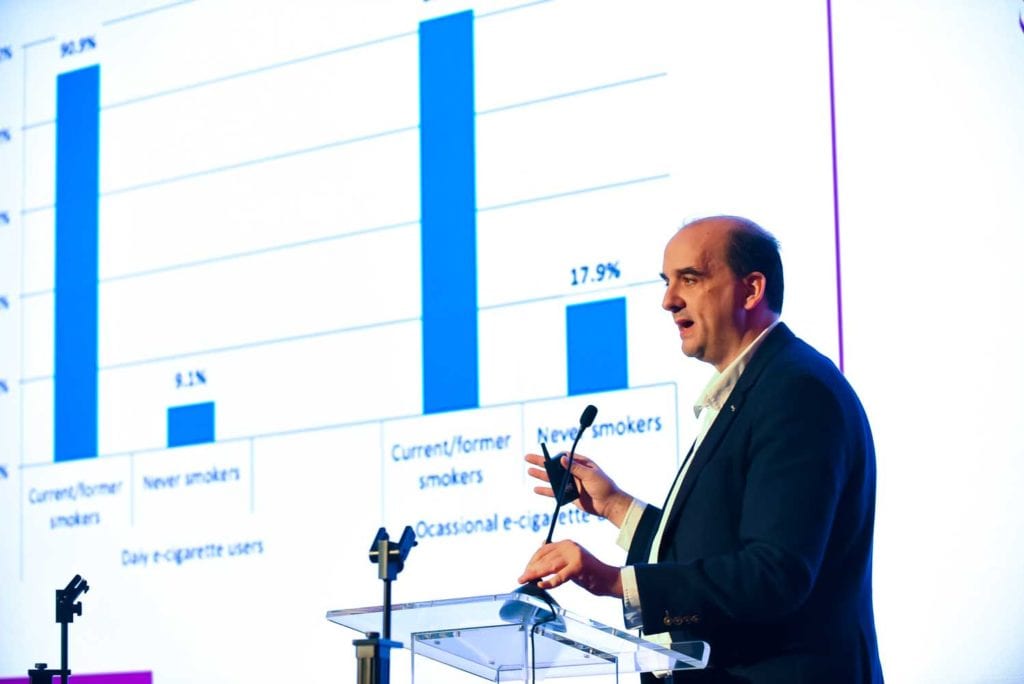
Electronic nicotine delivery systems (ENDS) are now the overriding method of choice for smokers who want to quit, says the report. Studies show users of flavored e-cigarettes are up to three times more successful.
The review highlights the work of David Levy, who developed the Smoking and Vaping Model, which allows researchers to calculate the life-saving potential if all adult cigarette smokers were to switch to nicotine vaping products. Applied to Canada, 130,000 deaths could be avoided between 2012 and 2052 if Canadian smokers switched to vaping. This would save 2.5 million life years.
The report points out that flavors are used to improve the efficacy of nicotine replacement therapy products, such as lozenges and gums, which feature on the World Health Organization’s list of essential medicines.
“Surely, if the WHO considers flavorings an essential anti-smoking tool in nicotine lozenges, the same should apply for consumer acceptance in nicotine vaping products” said Farsalinos.
“Vaping is already delivering results in Canada by helping long-term smokers who have struggled to quit to finally give up the habit. To misguidedly deny desperate smokers their best chance of quitting is needlessly putting their health at risk.”
The report recommends better access and affordability for quitting tools such as flavored vapes “through proportionate, risk-based regulation and robust monitoring.”
Concerns about underage use of vapes would be best addressed by focusing on youth access at the point of sale and the elimination of flavor descriptors clearly targeting the young, it adds.