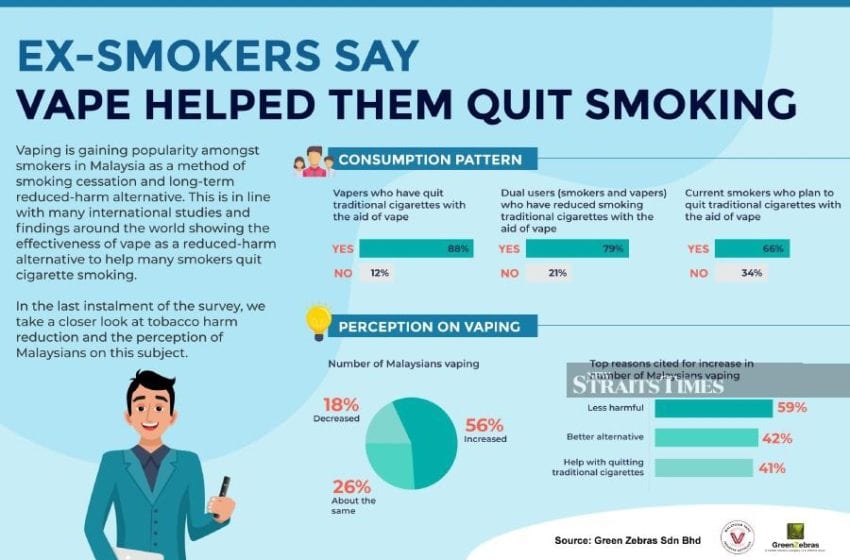
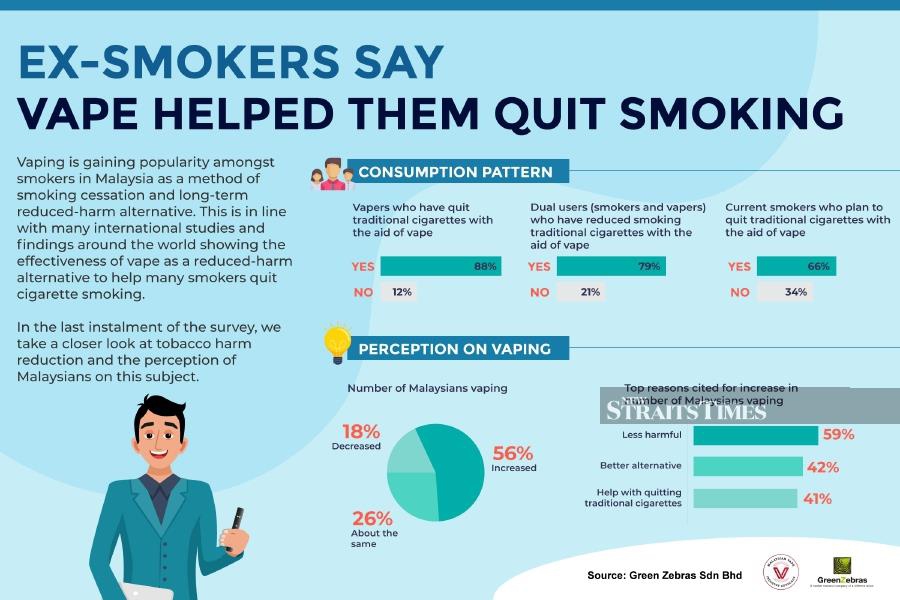
As part of a trial being led by the University of East Anglia, the U.K. National Health Service (NHS) will provide vaping devices and e-liquids to smokers coming to the emergency departments of five hospitals across the U.K. to help them quit.
Patients attending emergency departments in five hospitals in Norfolk, London, Leicester and Edinburgh will be offered a device, enough e-liquid supplies for a week and referral to local smoking cessation services alongside medical advice.
This will be followed up at one, three and six month intervals over a 30 month period to monitor success rates for those introduced to vaping compared to those only offered leaflets with details of local smoking-cessation services in the same trial.
“I welcome this trial being launched and the additional research, which will hopefully make it easier for people to quit smoking in the future,” said Norman Lamb, former health minister and former chair of the House of Commons science and technology committee.

I welcome this trial being launched and the additional research, which will hopefully make it easier for people to quit smoking in the future.
Norman Lamb, former health minister
“I am particularly keen to ensure that vaping is made available to people with mental ill health given continuing high smoking rates. It is very positive to have such a prominent trial funded by the National Institute for Health Research (NIHR) including clinical trials. I await the results with interest.”
The U.K. Vaping Industry Association (UKVIA) heralded the NHS’ decision as a landmark moment. “This is a hugely significant moment in the history of vaping and harm reduction,” said John Dunne, director general of the UKVIA.
“For the first time, following years of research and campaigning, we are finally at the point where the NHS looks to be fully embracing vaping and acknowledging its important role as the number one quit method.”
Dunne renewed his call to government to give vaping more opportunity to promote itself as a harm reduction alternative to smoking when it is due to review the Tobacco-Related Products Regulations in May.
“We have put forward the idea of using government-approved expert health claims on vaping products to encourage the remaining six to seven million smokers in the U.K. to switch as well as making sure that there are greater opportunities for the vaping industry to engage with smokers through marketing and advertising means, as current restrictions deter those who may have otherwise made the changeover,” he said.
“It is extremely important that hospital staff have the knowledge to advise smokers about vaping, including which devices to use, nicotine levels and flavors to opt for in order to support a successful quit.”