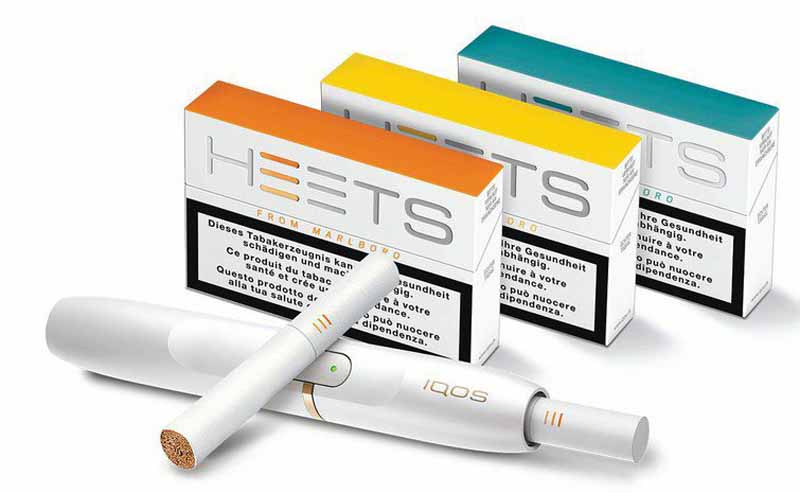
Philip Morris International said yesterday that it had stopped cigarette production at the Aspropyrgos factory of its Greek affiliate, Papastratos, which was now exclusively producing HEETS, the tobacco units used with its heated-tobacco product, IQOS.
‘This first full conversion of a cigarette factory is a landmark step in our vision of a smoke-free future where people who smoke switch from the most harmful form of nicotine consumption – cigarettes – to scientifically substantiated smoke-free alternatives,’ the company said in a note posted on its website.
‘The €300 million investment included the construction of three new buildings and the replacement of cigarette production lines with high-tech facilities capable of producing 10,000 smoke-free tobacco units per minute.
‘The conversion of the factory started in August 2017. The facility is expected to be fully operational by the end of 2018 and will create 400 new jobs.’
“This is a historic day for our company,” said André Calantzopoulos, PMI’s CEO. “Papastratos is the first of our factories to end cigarette production and fully shift to manufacturing our smoke-free alternatives.
“We will continue to convert existing sites and invest in new facilities to answer global adult smoker demand for better alternatives to cigarettes.
“We made a commitment to provide all people who would otherwise continue smoking with potentially less harmful products. The momentum around this revolutionary change for the benefit of the world’s 1.1 billion smokers, public health and society at large is growing, and we will continue working towards a smoke-free future.”
Along with PMI’s plant near Bologna, Italy, Papastratos is its second facility fully dedicated to manufacturing smoke-free products. PMI has announced plans also to transform, either fully or partially, its cigarette factories in Korea, Romania and Russia.
‘Since 2008, we have invested more than US$4.5 billion in scientific research, product and commercial development, and production capacity related to IQOS and other smoke-free products,’ the note said. ‘In 2017, over 70 percent of our global R&D expenditure and over 30 percent of our global commercial expenditure was allocated to smoke-free products.
‘We estimate that at the end of January 2018, nearly five million adult consumers around the world have already stopped smoking and switched to IQOS. Our ambition is that all those who would otherwise continue smoking abandon cigarettes and switch completely to scientifically substantiated smoke-free products as soon as possible. Appropriate regulatory policies and decisions can substantially accelerate the speed and magnitude of this historic change.’
Category: Harm Reduction

Turning the HEETS up

Modified risk application
Altria said on Tuesday that its smokeless tobacco business, US Smokeless Tobacco Company, had submitted to the US Food and Drug Administration a Modified Risk Tobacco Product application for its Copenhagen® Snuff Fine Cut moist smokeless tobacco product.
‘Altria aspires to be the US leader in authorized, non-combustible, reduced-risk products,’ the company said in a note posted on its website.
‘This action furthers Altria’s plan for submitting reduced-harm product applications with the FDA on a range of non-combustible tobacco products.
‘FDA will now undertake an administrative review to determine whether to accept the application for substantive review.’
Vaping masterclasses
A nationwide awareness and education campaign is due to be rolled out by the vaping sector across the UK in April.
VApril, which is being organised by the UK Vaping Industry Association (UKVIA), will be fronted by Christian Jessen, who is a medical doctor, television presenter and writer.
‘The initiative comes on the back of Public Health England’s (PHE) recent review into vaping which reinforced that it was 95 percent less harmful than smoking and revealed that it was one of the most successful ways to quit conventional cigarettes,’ UKVIA said in a press note.
‘The campaign will call upon the country’s smokers to Take the VApril Challenge, which will involve vaping masterclasses at specialist retail stores for smokers to learn about the different products and nicotine strengths that are best suited to a successful quit. As part of the initiative, the UKVIA has published a special education guide – Vaping to break the Smoking Habit.’
“I am always amazed and disappointed to hear that we still have seven million smokers in this country and, according to PHE, around 40 percent of them have never tried vaping,” Jessen was quoted as saying. “Furthermore, more than half of the population don’t realise that vaping is a fraction of the risk of smoking.
“That’s why I’m a committed supporter of the idea of a national vaping awareness campaign such as VApril to encourage smokers to take the first steps to quitting their habit. Already some 1.5 million vapers have given up smoking altogether, but more education is needed to ensure this figure keeps growing.”
John Dunne, a director of UKVIA, said VApril would be the largest campaign ever run by the vaping industry and reflected how far the sector had come in a relatively short time. “The challenge for the industry, government and the public health community is to get across the message that e-cigarettes are a very small risk compared to smoking and that nearly three million smokers are now vaping, with a significant number having switched over altogether,” he said. “VApril aims to be the starting point for more smokers to quit their habit.”
The VApril campaign has its own website at: www.vapril.org.
Speaking with smokers
The results of a survey carried out on behalf of the Foundation for a Smoke-free World has demonstrated that support for quit attempts can be better targeted by understanding the unique experiences of individual smokers.
The Foundation yesterday published findings from a global survey aimed at better understanding smokers, their experiences, and the challenges they face when they try to quit smoking. The survey highlights also their awareness regarding the harm caused by smoking and how their perceptions of cigarettes, alternative products and nicotine influence their motivation to move away from smoking.
The Foundation said the data would be used to shape the development of research to determine the best solutions to accelerate the end of smoking across diverse cultures and economic conditions.
The Foundation describes itself as an independent, private foundation formed and operated free from the control or influence of any third party. Philip Morris International provided the initial funding to the Foundation, which makes grants and supports medical, agricultural and scientific research to end smoking and its health effects, and to address the impact of reduced worldwide demand for tobacco.
The 2018 State of Smoking Survey included 17,421 current smokers, ex-smokers, and non-smokers from 13 countries: Brazil, France, Greece, India, Israel, Japan, Lebanon, Malawi, New Zealand, Russia, South Africa, the UK and the US. In parallel, a series of qualitative focus groups were carried out in seven countries, France, Greece, India, New Zealand, South Africa, the UK and the US, to give additional context to the quantitative results. The survey was conducted by Kantar Public, an integrated consulting and research agency, which was engaged by the Foundation.
In a press note, the Foundation said the primary findings were:- Smoking isn’t an isolated habit. Smokers consider it deeply integrated with their basic pleasures of life, such as eating, drinking, and socializing. Currently implemented cessation methods fail to take these into account, resulting in continued smoking.
- Smokers know that smoking is harmful to their health, and many consider themselves in poorer health than non-smokers, yet they do not actively engage with their healthcare providers or discuss effective cessation or reduced-harm solutions with them. The healthcare system needs to better engage with smokers, and medical providers need more effective tools to help smokers quit.
- There is confusion among smokers about the relative harms of smoking and less harmful alternatives. While people ‘smoke for the nicotine, but die from the tar,’ there is still considerable misperception about the risks of nicotine. This impacts their motivation to quit or try reduced risk alternative products.
“I hope this survey will jolt many of the world’s one billion smokers into action to stop smoking, spark a meaningful discussion on the deeply complex reasons so many people continue to smoke, and make clear the urgent need to develop more effective communications and interventions to help smokers quit or substantially reduce their risks,” said Dr. Derek Yach (pictured), president of the Foundation. “By better understanding key drivers behind why people start smoking, barriers to quitting, and motivations to stop, we can help reduce the negative health consequences for many who are trapped in the cycle of addiction to combustible tobacco products.
“The data demonstrates that by better understanding the unique experiences and struggles of the individual smoker, we can better support each individual’s quit journey. In this age of personalized medicine, it is only logical that we should stop treating the world’s smokers as one homogeneous group and start developing and embracing a wide range of solutions that allow individual smokers to select the method that works best for his or her situation and, more importantly, reduce the harm, disease, and death that is caused by smoking.”
Further findings from this study are available at:
https://www.smokefreeworld.org/sites/default/files/uploads/derek-yach-press-conference-presentation.pdf .
Italian e-cig initiative
An Italian anti-smoking organization is today staging an event at the EU Parliament during which experts and policymakers are due to discuss the benefits of electronic cigarettes.
In a press note issued through Business Wire, the Lega Italiana Anti Fumo (LIAF) said that while the use of e-cigarettes was growing continuously, worldwide, Europe was falling behind.
It was essential that Europe exploited the potential of this technology to reduce the impact of smoking on European public health, it said.
The LIAF is being hosted by the Italian MEP Giovanni La Via, who is a former chairperson of the parliament’s Environment, Public Health and Food Safety Committee.
It is intended that the event will provide the opportunity for an exchange of views on the potential of e-cigarettes to help reshape European health.
‘Though this is a very important public health issue, the discussion has been absent from the European agenda,’ the LIAF said in a press note.
‘With cigarettes killing more than half a million smokers a month globally and almost six million Europeans now using e-cigarettes to move away from smoking, providing scientific evidence to shift the balance from an “abstinence-only” agenda to a harm reduction strategy is vital in bringing down smoking deaths.’
“Europe is at a crossroads in the fight against smoking,” La Via was quoted as saying. “A growing body of evidence shows that there is a huge opportunity for public health in promoting the use of e-cigarettes to help people stop smoking. Health policymakers in Europe have a duty to provide the public with all of the facts on e-cigarettes, and to provide the best regulatory environment to help smokers quit completely.”
The press note said that recent reports had led key public health institutions to take a positive stance on e-cigarettes.
‘Well-respected bodies, such as Public Health England (PHE), Cancer Research UK and Action on Smoking and Health (ASH), recognise the potential of e-cigarettes to reduce the health effects of smoking,’ the note said. ‘PHE’s recent report on e-cigarettes (February 2018) concludes that vaping or using e-cigarettes are 95 percent safer than smoking tobacco.
‘Moreover, the report shows that while smoking rates among young people continue to fall, there is no evidence that e-cigarettes are a gateway to smoking. The same research found that e-cigarettes are used almost exclusively by those who have already smoked.’
Strung out on nicotine
The US Food and Drug Administration says that its release of an advance notice of proposed rulemaking (ANPRM) in respect of nicotine is a major step on the path to changing ‘dramatically’ the future of smoking in the US and saving millions of lives.
The ANPRM, Tobacco Product Standard for Nicotine Level of Combusted Cigarettes, is seeking public comment for consideration in developing a potential nicotine product standard.
The FDA believes that lowering nicotine to a minimally- or non-addictive level ‘could potentially save millions of lives, both in the near and long-terms’.
‘The ANPRM includes newly published estimates of one possible policy scenario for a nicotine product standard, including that approximately five million additional adult smokers could quit smoking within one year of implementation, compared to the baseline scenario,’ the FDA said in a note issued through its Center for Tobacco Products.
‘However, an even greater impact could be felt over time: by the year 2100, its estimated more than 33 million people – mostly youth and young adults – would have avoided becoming regular smokers. This could result in more than eight million fewer tobacco-caused deaths through the end of the century.
‘In July 2017, FDA Commissioner Scott Gottlieb, M.D., announced a new comprehensive plan that places nicotine – and the issue of addiction – at the center of the agency’s tobacco regulation efforts. As the cornerstone of the plan, the release of today’s [March 15] ANPRM is a major step on the path to dramatically changing the future of smoking in the United States and saving millions of lives.’
More information is at: https://www.fda.gov/TobaccoProducts/NewsEvents/ucm600955.htm?utm_source=Eloqua&utm_medium=email&utm_term=StratComms&utm_content=webemail&utm_campaign=CTPConnect%26News%26SOS%3A%20Special%20Announcement%20Nicotine%20ANPRM%20-%2031518.
Meanwhile, Gottlieb said in a statement that the ANPRM provided a wide-ranging review of the current scientific understanding about the role nicotine played in creating or sustaining addiction to cigarettes. It sought comments on key areas, as well as additional research and data for public review, as the FDA continued its consideration of developing a nicotine product standard.
‘We’re interested in public input on critical questions such as: what potential maximum nicotine level would be appropriate for the protection of public health?,’ the statement said. ‘Should a product standard be implemented all at once or gradually? What unintended consequences – such as the potential for illicit trade or for addicted smokers to compensate for lower nicotine by smoking more – might occur as a result? As we explore this novel approach to reducing the death and disease from combustible cigarettes, it’s critical that our policies reflect the latest science and is informed by the input we receive from our meetings with stakeholders, comments to the open public docket and future opportunities for comment.’
Gottlieb said also that the FDA’s plan demonstrated a greater awareness that nicotine, while highly addictive, was delivered through products on a continuum of risk, and that in order to address cigarette addiction successfully, it had to make it possible for current adult smokers who still sought nicotine to get it from alternative and less harmful sources.
‘To that end, the agency’s regulation of both novel nicotine delivery products such as e-cigarettes and traditional tobacco products will encourage the innovation of less harmful products while still ensuring that all tobacco products are put through an appropriate series of regulatory gates to maximize any public health benefits and minimize their harms,’ Gottlieb said. ‘This will be achieved through our ongoing regulatory work to develop several foundational rules, guidances, product standards and other regulations.’
Gottlieb said also that the FDA planned shortly to issue two additional ANPRMs: one to seek comment on the role that flavors – including menthol – played in initiation, use and cessation of tobacco products. ‘A second ANPRM will solicit additional comments and data related to the regulation of premium cigars.
‘At the same time we’re also jump-starting new work to re-evaluate and modernize our approach to the development and regulation of safe and effective medicinal nicotine replacement products such as nicotine gums, patches and lozenges that help smokers quit. This is a pivotal part of our overall public health approach.’
Gottlieb’s statement is at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm601039.htm.
Transforming tobacco now
The emphasis was on ‘transforming tobacco’ when British American Tobacco yesterday published its Annual Report and Sustainability Report.
Both of the reports are entitled ‘Transforming Tobacco’, reflecting ‘the company’s commitment to transform tobacco by offering an unrivalled suite of potentially reduced-risk products that address the varied preferences of today’s consumers’.
In a note posted on its website, BAT said the reports clearly set out its ambition to lead and shape the transformation of the industry by achieving a triple win: ‘for consumers – who will be offered a range of potentially safer choices; for society – who could benefit from real progress in tobacco harm reduction; and for shareholders – who will own an even more sustainable and profitable business’.
The note said that BAT’s existing combustible portfolio was now complemented by a wide range of potentially reduced-risk products, including next generation products (NGPs), comprising vapor and tobacco heating products (THPs), as well as oral tobacco and nicotine products, such as snus and moist snuff.
“We recognise that the tobacco and nicotine industry has entered a dynamic period of change and we are committed to leading this transformation,” chief executive, Nicandro Durante, was quoted as saying. “Increased public health awareness, new societal attitudes and rapid developments in new technologies have all combined to create a unique opportunity to accelerate the delivery of our long-held ambition to provide our consumers with less risky tobacco and nicotine choices.
“These two important reports out today, clearly outline our strategy to transform tobacco by building our business based on outstanding products, informed consumer choice and a potentially reduced-risk portfolio. Put simply: more choice, more innovation, less risk.”
BAT said its commitment to the important role these potentially reduced-risk products would play was reflected in its aims to generate more than £1 billion revenue from NGPs in 2018 and to increase this figure to £5 billion by 2022.
Since 2012, together with Reynolds American Inc., BAT had invested about US$2.5 billion in developing and commercialising its range of NGPs.
“Transforming tobacco isn’t just about harm reduction, though,” said Durante. “To respond to evolving challenges and opportunities, we need to continue to focus on the other key areas of our sustainability agenda, including sustainable agriculture and farmer livelihoods, and corporate behaviour. These are fundamental imperatives that set the foundations for our business for years to come.”
Smoking frozen out
An Icelandic doctor has credited vaping with contributing to a dramatic decline in the number of smokers in Iceland, according to a story by Paul Fontaine for grapevine.is.
Guðmundur Karl Snæbjörnsson described vaping as “a great blessing” for Icelanders’ health.
In 2014, 14 percent of the population or 35,000 people self-identified as smokers, figures that had fallen to nine percent and 22,000 by 2017.
The 37 percent drop in the number of smokers was attributable in part to an increase in vaping, Guðmundur told reporters.
Cigarette sales had dropped by 50 percent from 2008 to 2017, while vaping had been on the rise, and now, about 20,000 Icelanders vaped daily or less frequently.
“Smoking has been falling like a rock like we’ve never seen before,” Guðmundur was reported to have said. “The biggest contributing factors have been mouth-tobacco and vaping, which have clearly been wiping smoking out.”
Iceland currently has no clearly defined laws about the contents, sale and distribution of vaping products.
Although a bill was introduced last year that set limits on e-fluid strength and quantities; that bill was strongly opposed by vape shop owners and ended up dying in committee.
Vive la différence
Russia’s Minister of Industry and Trade, Denis Manturov, has said that Russia is preparing to categorize vaping products and tobacco products differently, and therefore to regulate them separately, according to a story by Diane Caruana for vapingpost.com
Manturov said the Russian government had taken the initiative to place vaping devices into a separate category because they were radically different from traditional cigarettes and tobacco.
This motion will be commended by many public health experts, who have been pointing out that any product regulations should be related to the risk of the product.
“Electronic devices are safer,” said Manturov. “Many experts, including Western experts, even articulate a figure: electronic means of nicotine delivery are 95 percent less harmful than conventional cigarettes.”
The minister said also that Russia’s Ministry of Health was onboard with the proposal to categorize the two products separately, and was urging smokers to switch to the safer alternatives.
Evidence building
A clinical study conducted by scientists at British American Tobacco has revealed that when smokers switch completely from cigarettes to the heated-tobacco product, glo, their exposure to certain cigarette smoke toxicants is significantly reduced, in some cases to levels comparable to those seen in smokers who quit smoking completely.
In a press note issued today, BAT said these results added to evidence suggesting that glo may ‘have the potential to be substantially reduced risk compared to smoking conventional cigarettes’.
‘Because glo vapor has lower levels of toxicants than cigarette smoke, it should in principle expose consumers to much less toxicants,’ the note said. ‘The results of this study indicate that this is indeed the case.’
The clinical study was conducted in Belfast, UK, over seven days and involved 150 people, all of whom were smokers for at least three years prior to enrolment.
‘For the first two days, study participants continued to smoke as normal and their urine was collected to measure levels of chemicals. Blood and breath were also collected for analysis,’ the note said.
‘For the next five days, participants were randomly allocated to either continue smoking, switch to using a THP [tobacco heating product] or quit smoking. Urine, blood and breath samples were again collected for analysis.
‘Exposure to certain smoke toxicants was determined by measuring the levels of certain chemicals in the urine. These could be the toxicants themselves or their metabolites – which is what the body breaks it down into – called biomarkers of exposure. Toxicants measured included those identified by the World Health Organization as being of concern in cigarette smoke.’
The results were said to have shown that the concentration of certain chemicals in the urine was reduced in smokers who switched to glo. In some cases, these reductions were the same as those observed in the smokers who quit.
“These results are very encouraging,’ said Dr. James Murphy, head of reduced risk substantiation at BAT. “The next step will be to determine whether this reduction in exposure translates to a reduced biological effect, and in turn a reduction in adverse health effects for those smokers who switch completely to glo.”
BAT said that future clinical studies would test for markers of biological effect, such as cholesterol levels or heart rate – measurements that give an indication of general health. A reduction in biomarkers of biological effect could suggest that a reduction in exposure is having a positive impact on reducing the adverse health risks of smokers who switch completely.
“The results of one test are important,” said Murphy, “but it is the combination of the results of many different tests that start to give us a real feel for the bigger picture and the potential for glo to be reduced risk compared to a conventional cigarette.”
The results of the clinical study are being presented today at the annual conference of the Society of Toxicology in San Antonio, Texas, US.