Public Health England (PHE) said yesterday that going ‘cold turkey’ was the least effective way to quit tobacco smoking.
In a note to mark No Smoking Day, PHE said that over 58 percent of smokers still tried to quit without using an aid, despite this being the least effective way.
‘A Public Health England (PHE) report highlights that public misunderstanding of the harmfulness of nicotine-containing products, such as nicotine replacement therapy (NRT) and e-cigarettes, may be linked to inaccurate and confused perception of the risks of nicotine,’ the note said.
‘The risks of nicotine use are likely to be very low or negligible. NRT is safe and licenced for use in pregnancy and for people with cardiovascular disease. And there is now wide international consensus that e-cigarettes are far less harmful than smoking. It is the cocktail of deadly chemicals in cigarette smoke, including tar and carbon monoxide, which causes almost all of the harm of smoking.
‘Four in 10 smokers and ex-smokers incorrectly think that nicotine in cigarettes is the cause of most of the smoking-related cancer. Understanding of the harms of nicotine among the general population is similarly poor…’
The PHE note is at: https://www.gov.uk/government/news/four-in-10-smokers-incorrectly-think-nicotine-causes-cancer.
Category: Harm Reduction

Nicotine misunderstood

Reducing risk
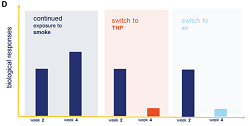
A new laboratory-based study has shown that when airway cells damaged by cigarette smoke are exposed instead to vapor from the heated-tobacco product, glo, some of the biological effects caused by the smoke exposure are reversed.
Basically, subsequent exposure of cigarette-smoke-impacted cells to glo vapor had a similar effect to their subsequent exposure to air – the cells were able to maintain their ability to repair themselves.
In a press note, British American Tobacco said that the vapor produced by glo contained about 90-95 percent less of certain toxicants than did cigarette smoke.
The company said that previous studies had shown that the biological impact of glo vapor on cells tested in the laboratory was much less than the impact of cigarette smoke on similar cells. But, it added, the reversibility of the damage following switching to a product such as glo had not been extensively studied.
“Products like glo are very new, so understanding the biological impact of vapor from glo and how that compares to cigarette smoke is a core component of our scientific research,” said Dr. James Murphy, head of reduced risk substantiation at BAT.
In the study, human airway cells were exposed repeatedly to either cigarette smoke or vapor over four weeks. For the first two weeks, the lung tissue was exposed to cigarette smoke for 15 minutes at a time, three times a week. The exposed tissue was then split into three groups: one group continued to be exposed repeatedly only to cigarette smoke for a further two weeks; a second group was exposed repeatedly to glo vapor and the third group was exposed only to air. The results obtained were then compared to results obtained by exposing airway tissue only to air for the full four weeks.
‘The results show that switching completely to glo after two weeks of repeated exposure to cigarette smoke reversed some of the biological impacts of the smoke,’ the press note said. ‘Significant reductions were observed in the amount of certain molecules produced in response to inflammation, for example, in comparison to that seen in lung tissue exposed to cigarette smoke for the full four-week period.’
BAT said the results added to evidence suggesting that glo might have the potential to be reduced risk compared to conventional cigarettes.
“We have developed a suite of tests to assess our next generation products, because we know it is by taking the results of all these tests together that gives us a real feel for the bigger picture and the potential for glo to be reduced risk compared to a conventional cigarette,” said Murphy.
The results are being presented today at the annual conference of the Society of Toxicology in San Antonio, Texas, US.
Reducing risk reduction
A smokers’ lobby group has criticised the UK government’s plan, revealed yesterday, to introduce an excise tax on heated tobacco products.
According to the government, the duty on these products will be based on the weight of tobacco in the product.
“Heated tobacco may not be as safe as electronic cigarettes but current evidence suggests there is almost certain to be a reduction in risk for cigarette smokers,” said Simon Clark (pictured), director of Forest [Freedom Organisation for the Right to Enjoy Smoking Tobacco].
“Why would any government want to undermine the future of a product that may encourage smokers to quit voluntary and without coercion?”
Clark pointed out that many smokers who tried electronic-cigarettes found they didn’t like them. The attraction of heated tobacco was that it filled the gap between combustible cigarettes and e-cigarettes, which don’t contain tobacco.
“Heated tobacco products are still in their infancy,” he said. “Adding excise duty will almost certainly deter many smokers from switching to a potentially safer device.”
The law is a butt
The New Zealand government has been criticised for going ahead with the prosecution of a tobacco company while acknowledging that the law on which the case is based needs to be updated.
In a press note, the New Zealand Taxpayers’ Union (NZTU) said that the 1990 legislation, written for chewing tobacco, might inadvertently ban new heat-not-burn products – a matter that was currently before the courts.
Citing a Stuff report, the NZTU quoted the Ministry of Health’s prosecutor as saying that the legislation needed to be brought up to date and that these changes were “in train”.
“The current law is cruel – smokers are taxed into poverty, and then told that alternatives, such as e-cigarettes and heat-not-burns, are illegal,” said the NZTU’s executive director Jordan Williams. “The Ministry acknowledges the law is outdated, so why proceed with court action?
“Every e-cigarette retailer and consumer has reason to worry when the Ministry of Health is taking criminal prosecutions while even acknowledging that the law is an arse.
“With the Ministry making this acknowledgement, the only reason the Government has left to not change the law is its addiction to tobacco taxes.”
A New Zealand Herald story reported on here yesterday, said that the court case involved Philip Morris defending two charges over the sale of its HEETS tobacco sticks that are used in its IQOS electronic heated-tobacco device.
If the ministry proved that this product was for oral use, but not smoking, that would make its sale illegal under current New Zealand law.
Health ministry prosecutor Sally Carter was said to have told the Wellington District Court that the issue came down to legal fine print.
“It’s the heat sticks that contain tobacco, and there’s no problem that this product contains tobacco,” she said. “The real problem is whether this product falls within the Smokefree [Environments] Act 1990.
“Significantly, because of the way the Act is structured there are issues whether in fact the product is a smoking issue, and a smoking product.
“The definition of ‘to smoke’ means that the product needs to be ignited.”
Philip Morris was said to be defending the charges, arguing that HEETS comprise a smoking product, even though the tobacco in them is heated, not burned.
E-cigs given a hearing
British American Tobacco’s chief scientific officer yesterday told a UK parliamentary committee that policymakers should maximize the potential of electronic cigarettes to provide an alternative to combustible cigarettes.
In his evidence to the Science and Technology Committee, which is holding an enquiry into e-cigarettes, Dr. Chris Proctor (pictured) focused on three key aspects of the vaping category.
Firstly, he said, there was a need for a broad range of products: a need to understand that it was not a “one-size fits all” situation. ‘Over the last 4-5 years we have seen strong growth in the e-cigarette category, but different consumers have different needs,’ Proctor was quoted in a BAT press note as having told the committee. ‘While e-cigarettes work for many, we need to recognise that other products, such as tobacco heating products, can also play a part. Today, at BAT we have an unrivalled range of exciting and innovative products across the potentially reduced risk categories including industry-leading products in vapor, tobacco heating products, oral tobacco, tobacco-free nicotine pouches, and moist snuff.’
Secondly, Proctor said, there was a need to address marketing restrictions because BAT believed there was a lack of public understanding about these new products that could be holding the category back. It was crucial, he added, that there was appropriate regulation in place to allow sensible marketing freedoms to give consumers the information they needed.
Thirdly, there was a need for greater focus and emphasis on product standards and quality. There was a need for quality and product-safety standards that could become the benchmark for the industry and regulators. It was only with universal standards that consumers would get the quality assurances they rightly needed.
After his committee presentation, Proctor issued a statement that welcomed the opportunity to give evidence before the committee and that underlined the need for officially-recognized product standards. “For many years, and based on our own extensive and continued research, testing and development of our products, we have highlighted that smokeless products – like vaping and tobacco heating products – can be a potentially reduced risk alternative to smoking,” he said. “This is why we have, and continue to, invest heavily in a portfolio of innovative, high-quality next-generation products, accelerating our ambition to transform tobacco.
“The UK is the largest vaping market in Europe and we believe this is largely due to its pragmatic and progressive approach to this category. However, there is still more to be done. Whilst it is great that there is increasing debate around these new products and more supportive science, we need to recognise that not all products within the category are the same. It is crucial that there is appropriate regulation in place to ensure high product standards and quality, whilst also giving sensible innovation and marketing freedoms. Only with these industry standards, will consumers get the information and quality assurances they rightly need.”
E-cigs work without nicotine
The Cancer Council Australia (CCA) has urged smokers to be cautious about overseas research claiming that electronic cigarettes could help them kick their habit, according to a story by Annie Lewis for the Wagga-based Daily Advertiser.
The CCA’s director of advocacy Paul Grogan said that in Australia the jury was very much still out on the health effects of vaping.
Grogan said that while he welcomed international research, such as a recent study by Public Health England, Australian researchers were looking at the “whole picture” before making a determination.
“There is convincing evidence, including a meta-analysis in a respected American journal, showing a clear association between e-cigarette initiation and long-term smoking,” he said.
Grogan said too that Australia needed to keep in mind how well it had done in reducing rates of smoking, and how the biggest investor in the e-cigarette sector was the tobacco industry.
He said that the position of the CCA was very open to evidence-based research, but the emphasis was placed on getting all the facts.
Meanwhile, smokers seem to be taking a different view. The Wagga business, Vaped, was said to have almost sold out of stock in its first week.
“The success rate is big; it definitely works for all our customers,” Vaped owner, Lance Carr, was quoted as saying.
“We do get a lot of repeat customers that come back saying they haven’t smoked because the e-cigarette is working.”
Carr said that while some users bought their own nicotine, that wasn’t the case for the majority.
“We don’t encourage the use of nicotine because it’s illegal to sell it,” he said.
“For a heavy smoker nicotine is recommended but I was surprised when we first opened; I thought it would have been that the majority needed nicotine in their liquids but it’s the opposite.”
Dithering over HNB
The government in South Korea seems to have run into problems in its quest to measure the risks and relative risks of using heat-not-burn (HNB) products in place of traditional cigarettes.
According to a story in The Korea Herald, in July, the Ministry of Food and Drug Safety said it would conduct safety tests on HNB products and come up with an objective analysis of the health risks posed by using such devices. At that time, it said it would focus on the levels of nicotine and tar delivered by the HNB products’ tobacco sticks.
But the ministry has yet to release the test results and now says that a new system is needed to test the products.
“Because this is a relatively new product, we may have to create new regulations or systems to deal with e-cigarettes,” a ministry official was quoted as saying.
For instance, due to continued public calls for verification of the risk posed by HNB devices, the government was considering introducing a law that stipulated that tobacco companies should release a list of HNB tobacco-stick components.
But tobacco firms said the government’s idea might not be feasible.
“If we hand in a list of e-cigarette ingredients, it will be more than several pages long, because there are so many kinds of chemicals inside the product,” a tobacco-company official was quoting as telling the Herald. “Unlike a cigarette, it is also hard to measure the content inside the e-cigarette stick because those sticks vary in sizes and grams, depending on the device that goes with them. This is also why we weren’t able to release the components list.”
Some experts were said to claim that it wasn’t important how much tar, nicotine and toxicants were delivered by HNB cigarettes, because the important factor was how often a person used such devices.
Nevertheless, a lot of smokers seem to have made up their own minds. In January, sales of HNB cigarettes accounted for 9.1 percent of South Korea’s tobacco market.
Ghanaians battling NCDs
The Ghana Non-Communicable Disease Alliance (GNCDA) is worried about what it sees as an upsurge of alcoholism and ‘excessive smoking’ in the country, especially among young Ghanaians, according to a story broadcast by the Ghana Broadcasting Corp.
Speaking at the official launch of the GNCDA in Accra, the group’s vice chairperson, Adams Ebenezer, said that exposing children to these harmful products was a violation of their human rights.
The GNCDA is a newly-formed network of Non-Governmental Organizations that was officially registered this year with the mission of becoming a leading organization contributing to reducing NCD-related deaths and disabilities, and improving the quality of lives of people living with NCDs.
It aims to support and complement the government.
Shisha tobacco had become a lifestyle product among many of the country’s young people, who were ignorant about the danger posed by the use of this product, Ebenezer said. Research had shown that a session of shisha smoking was the equivalent of smoking 100-150 cigarettes.
Ebenezer said the only known way of reducing health issues such as lung cancer, oral cancer, neck cancer, heart disease, hypertension, obesity and extreme poverty was to exercise absolute control over tobacco use, exposure to tobacco smoke and alcohol.
E-cigs as treatment aid
Researchers in New Zealand say that more thought should be given to the use of electronic cigarettes in hospitals because the use of these products can lessen the stress of patients’ treatment, according to a stuff.co.nz story.
Dr. Penelope Truman of Massey University’s School of Health Sciences said its research had found that e-cigarettes could aid patients battling alcohol addiction and patients admitted to psychiatric units.
More than 40 patients at Kenepuru Hospital in Porirua were studied in two cohorts between 2013 and 2016.
Truman said there were similar reductions in smoking when patients were offered e-cigarettes or conventional nicotine-replacement therapies, such as patches or gum.
The trial had shown there were no “significant” problems with patients using e-cigarettes in a hospital setting, and that these products could be beneficial.
On the other hand, going outside for a smoke could create a lot of problems for patients and the staff trying to care for them.
Alcoholics who were also smokers were offered the option of using an e-cigarette as well as, or instead of, conventional nicotine replacement therapy to stop smoking while in hospital.
The e-cigarettes proved to be more popular than standard therapy, and were at least as effective, Truman said.
“This is only a little trial, but I think it does raise some questions,” she said. “A lot of people are going to have to reconsider how they feel about people vaping around them, because it’s going to become increasingly popular.”
The full story is at: https://www.stuff.co.nz/national/health/101689960/ecigarettes-could-have-a-positive-impact-in-hospital-environments-research-finds.
Another glowing report
A clinical study conducted by scientists at British American Tobacco has revealed that when smokers switch completely from cigarettes to the tobacco-heating product (THP) glo, their exposure to certain cigarette smoke toxicants is significantly reduced, in some cases to levels comparable to those seen in smokers who quit smoking completely.
The company points out in a footnote that the results of the study do not necessarily mean that glo is less harmful than are other tobacco products.
It says, however, that the results add to ‘evidence suggesting that [using] glo may have the potential to be substantially reduced risk compared to smoking conventional cigarettes’.
A BAT press note said that glo was designed to heat rather than burn tobacco, which meant that it did not produce smoke, and that certain toxicants associated with tobacco combustion were substantially reduced. Previous studies had revealed that toxicant levels in the vapor from glo were about 90-95 percent lower than they were in cigarette smoke.
“Products like glo are very new and consumers and regulators alike understandably want as much information as possible about them,” said Dr. James Murphy, head of reduced risk substantiation at BAT. “Understanding how vapor from glo compares to cigarette smoke is, therefore, a core component of our scientific research. Clinical studies, which are studies involving real people, are an extremely important component of that.”
The press note said that because glo vapor had lower levels of toxicants than did cigarette smoke, it should in principle expose consumers to much lower levels of toxicants.
The results of this study, which were due to be presented on Saturday at the annual conference of the Society for Nicotine and Tobacco Research in Baltimore, Maryland, US, indicated that this was the case.