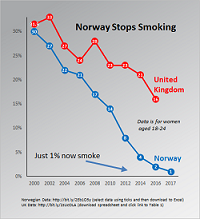
The uptake of snus in Norway is being credited with almost eliminating cigarette smoking among young people living there.
In a note published on its website today, the New Nicotine Alliance (NNA) said that government figures showed the incidence of smoking among women aged 16-24 was down from 30 percent in 2001 to 0.1 percent, while the incidence of smoking among young men was down from 29 percent to three percent.
The NNA said that the fall in smoking among Norway’s young people did not appear to be the result of their switching to vaping because nicotine-containing electronic-cigarettes were only now being legalised.
A more likely explanation seems to be presented by a sharp increase that has occurred in the use of snus. During 2008-14, snus use among young women grew from five percent to 14 percent.
In neighbouring Sweden, where snus is also legal, 20 percent of the population use snus and there the adult smoking rate has fallen to five percent.
Last month the European Court of Justice held a hearing on whether the EU ban on snus outside Sweden should be lifted, an action that has been supported by the NNA.
Its trustee Professor Gerry Stimson was quoted as saying that any reasonable person looking at the spectacular graph for smoking among young Norwegians would be struck by how the fall accelerated after snus became available in 2002.
“This is no fluke,” he said. “The end of smoking is in sight in Norway and Sweden as people choose far safer snus instead.
“So reasonable people will ask why the UK government decided to urge the European Court of Justice to maintain the snus ban in the rest of the EU.”
His comments were echoed by the smoking-substitutes expert Dr. Konstantinos Farsalinos who said there was absolutely no doubt that access to snus in Sweden and Norway had played a crucial role in the rapid reduction of their smoking rates.
Category: Harm Reduction

EU in denial over snus
Vaping key to quitting
Vaping poses only a small fraction of the risks of smoking and switching completely from smoking to vaping conveys substantial health benefits, according to a new Public Health England (PHE) electronic-cigarette evidence review.
The report, which was undertaken by leading independent tobacco experts, provides an update on PHE’s 2015 review.
It covers e-cigarette use among young people and adults, public attitudes, the impact on quitting smoking, an update on risks to health and the role of nicotine. It also reviews heated tobacco products.
PHE lists the report’s key findings as:
* E-cigarettes could be contributing to at least 20,000 successful new quits per year and possibly many more;
* E-cigarette use is associated with improved quit success rates over the last year and an accelerated drop in smoking rates across the country;
* Many thousands of smokers incorrectly believe that vaping is as harmful as smoking; around 40 percent of smokers have not even tried an e-cigarette;
* There is much public misunderstanding about nicotine. Less than 10 percent of adults understand that most of the harms to health from smoking are not caused by nicotine;
* The use of e-cigarettes in the UK has plateaued over the last few years at just under three million;
* The evidence does not support the concern that e-cigarettes are a route into smoking among young people. Youth smoking rates in the UK continue to decline. Regular use is rare and is almost entirely confined to those who have smoked.
PHE’s evidence review comes a few weeks after a US National Academies of Sciences, Engineering and Medicine report on e-cigarettes found that, based on the available evidence ‘e-cigarettes are likely to be far less harmful than combustible tobacco cigarettes’.
Professor John Newton, Director for Health Improvement at PHE said that smoking led to someone being admitted to hospital every minute in England, and that there were about 79,000 smoking-related deaths a year in England alone.
“Our new review reinforces the finding that vaping is a fraction of the risk of smoking, at least 95 percent less harmful, and of negligible risk to bystanders,” he said. “Yet over half of smokers either falsely believe that vaping is as harmful as smoking or just don’t know.
“It would be tragic if thousands of smokers who could quit with the help of an e-cigarette are being put off due to false fears about their safety.”
David O’Reilly, British American Tobacco’s group scientific and R&D director, welcomed the report.
“We welcome this latest report from Public Health England which reiterates their view that e-cigarettes are less harmful than smoking; that accurate information is needed about these new products; and that the evidence does not support that e-cigarettes are a gateway to smoking, and may in fact be an important tool to help people quitting,” he said. “It’s positive to see that for the first time they’ve also referenced tobacco heating products [THPs] – and how the available information suggests that these may also be considerably less harmful than traditional cigarettes.
“The report noted that there is significant public misunderstanding about risks associated with vaping and this has coincided with a plateauing of use of e-cigarettes in the U.K. We believe that this lack of understanding could be holding back this important consumer category – consumers and regulators need accurate information to provide them with the facts they need on the potential safety profile of these products. We believe the industry, public health and regulators have a role to play in providing accurate and robust information to support this important category.
“The science we’ve done on our products, across e-cigarettes and tobacco heating products, is pointing in the direction of these being a potentially safer alternative to cigarettes. We all agree that more long-term data is needed and, in line with this, at BAT, we continually assess our products, with many long-term studies currently underway across vapour and THP with our Vype and Glo brands respectively.
“Tobacco harm reduction is a critical part of our company’s strategy. We are committed to offering consumers a choice of high quality, innovative and inspiring alternative products with reduced risk potential, from vapor to THP. With increasing evidence in support of e-cigarettes, as an option for smokers looking for potentially safer alternatives, it is crucial that there is appropriate regulation in place to give consumers the information they need. It is imperative that regulations ensure high product quality and give sensible innovation and marketing freedoms, whilst also ensuring that these products are not available to youth.
“We’ve invested $2.5 billion in this important consumer category over the last six years and our commitment to the future is larger still as we seek to transform tobacco. Our quest to offer more alternatives to cigarettes, with harm reduction potential, could transform tobacco for consumers, regulators and society.”

A glowing report on vapor
Scientists at British American Tobacco have reported that they observed changes in just two genes when human airway tissue was exposed to vapor from the company’s glo tobacco heating product (THP), whereas thousands of gene changes were observed in tissue exposed to cigarette smoke.
They pointed out, however, that these results do not necessarily mean that the use of glo is less harmful than is the use of other tobacco products.
‘The impact on tissue exposed to glo vapor was minimal and more comparable with that of air when tested in laboratory conditions,’ according to a BAT press note.
‘These results add to evidence suggesting that glo has the potential to be substantially reduced risk compared to smoking conventional cigarettes.’
As part of the press note, Dr. James Murphy, head of reduced risk substantiation at BAT was quoted as saying that products such as glo were new, and that consumers and regulators wanted as much information about them as possible. That was why testing the impact of glo vapor compared to that of smoke was so important.
In this case, scientists were observing gene expression, which could give an indication of whether exposure to an aerosol, such as smoke or glo vapor, had had particular toxic effects.
BAT’s results clearly showed that cigarette smoke triggered a robust gene expression response, while exposure to vapor from glo had very limited impact on gene expression. Murphy said there was a striking difference.
In the recent study, scientists at BAT used human cells grown in the laboratory to test the impact of glo vapor and compare it to the impact of smoke and air.
The tissue (MucilAir™) is made up of human cells that grow in the laboratory to create a 3-dimensional structure that mimics the natural structure and characteristics of the living human airway. The resulting tissue is, for example, capable of producing mucus, as in the living airway, and it is covered in hair-like projections called cilia, which are used to expel inhaled dust from the respiratory system.
‘Using a robot that mimics how consumers use their products, this tissue was exposed to air, smoke from a reference cigarette (3R4F), or vapor from glo continuously for one hour,’ the BAT note said. ‘Then, to measure the cell response, the scientists mapped the genes that were switched on and off at 24 hours and 48 hours after the one-hour exposure.
‘This involves breaking open the cells and the cell nucleus to get at the genetic material inside it. The material is then studied to determine what genes are impacted.’
“Our technology is state-of-the-art,” said Murphy. “We have the capability to profile the activity of tens of thousands of genes simultaneously, providing more information than ever before on the genetic profile of exposed cells.”
‘Results show that cigarette smoke triggered thousands (2809) of changes in the expression of genes strongly involved in the development of lung cancer, inflammation and fibrosis,’ the note said. ‘In contrast, only two genes were affected by exposure to glo vapor.
‘These results, which are published in the journal Scientific Reports (doi: 10.1038/s41598-018-19627-0) add to evidence that glo vapor may cause less damage to cells as compared to cigarette smoke. Future studies will look at the impact on human tissues of more intense and longer exposure to this vapor.
‘Previous research conducted by British American Tobacco has shown that glo vapor contains around 90-95 percent less toxicants compared to cigarette smoke from a reference cigarette, in terms of the priority list of nine toxicants that the World Health Organization recommends reducing in cigarette smoke.’
Pod-device is guaranteed
Imperial Brands said yesterday that it had launched, via its subsidiary Fontem Ventures, a new product into the fastest-growing segment of the e-vapor category in the US, which comprised pod-based devices.
‘The myblu™ device is a pod-based system that provides consumers with power, performance and simplicity in a compact and user-friendly design,’ Imperial said in a note posted on its website.
‘Myblu can be fully charged in only 20 minutes and is supported by a lifetime device warranty, myCARE™.’
Antoine Blonde, general manager at Fontem Ventures USA, was quoted as saying the device would significantly enhance the vaping experience for consumers.
“Our constant quest for innovation and improvement is at the heart of blu as a brand,” said Blonde. “We believe that myblu™ will set a new benchmark in the e-vapour category.
“Consumer feedback has been very positive and we’ve sold out of product pre-orders faster than ever before.”
Imperial said that myblu™ launches were scheduled to follow shortly in other markets, including those in the UK, France and Italy.
Study is a ‘wakeup call’
A new Tel Aviv University (TAU) study published in Addiction finds that eight out of 100 smokers who take smoking cessation medications will have benefited from taking such medications after one year’s time, according to a story in medicalxpress.com.
The researchers conclude that this is a low rate of success that should encourage policymakers to try to find better methods to help smokers quit, and to prevent young people from taking up smoking.
“By the end of the first year of intervention, only eight out of 100 smokers will have abstained from smoking due to the smoking medication,” said lead researcher Dr. Laura J. Rosen of the School of Public Health at TAU’s Sackler Faculty of Medicine.
“This study is particularly important in Israel, where 22.5 percent of adults smoke and the rate of smoking is not declining. While the Israeli national healthcare system offers a strong package of aid to smokers who want to quit, there is no permanent funding for other tobacco control strategies.”
The scientists used meta-analysis to combine the results of 61 randomized controlled trials involving some 28,000 participants who took the first-line US Food-and-Drug-Administration-approved smoking cessation medications bupropion (Zyban), nicotine replacement therapy (NRT) or varenicline (Chantix/Champix). In all of the trials, participants were randomized either to an intervention group, which received smoking cessation medications, or to a control group, which did not receive active medications. Most of the trials also featured some form of counseling in addition to the medication.
“Less than 40 percent of those receiving the medications continued to abstain from smoking after three months, about 25 percent had still quit after six months, and about only a fifth — 20 percent — remained abstinent after a full year,” Rosen says. “Importantly, 12 percent of those who did not receive active medication continued to abstain from smoking after one year.
“Because benefit is calculated by starting with the quit rate among those who received the medication, and subtracting from the percentage who quit in the groups which didn’t receive the medication, just eight percent of smokers who received smoking cessation medications continued to benefit from the drugs after one year.”
According to Rosen, this study differs from previous meta-analyses in that it examines the relative success of quitting over different time periods (three, six and 12 months) and the overall decline in benefits from the medication over time.
“This study is a wakeup call for policymakers everywhere and for physicians who treat smokers,” Rosen concludes. “Much more needs to be done to reduce tobacco use and its enormous toll on the population. We applaud current efforts by the FDA to develop more beneficial forms of medicinal nicotine for smokers who want to quit. Policymakers should use all possible means to prevent young people from starting to smoke. Prevention of entry into the cycle of addiction is the best possible medicine.”
The full story is at: https://medicalxpress.com/news/2018-01-benefits-cessation-medications-diminish.html
E-cig rules challenged
US Food and Drug Administration rules threatening the survival of the US’ independent electronic-cigarette industry could be overturned if a new lawsuit filed by the non-profit Pacific Legal Foundation proves successful, according to an op-ed piece by Guy Bentley for the Washington Examiner.
Bentley said that the FDA’s ‘deeming rule’, which was issued in 2016, brought e-cigarettes under the agency’s regulatory control, subjecting them to the same kinds of marketing restrictions as those governing tobacco cigarettes.
Even to get approval for sale, vapor products would have to be taken through a prohibitively expensive pre-market approval process, and Bentley predicted that most e-cigarettes would disappear from the market by 2022, when product applications were due.
And the manufacturers of those products that did get through the application process would face an even greater battle to be allowed to make straightforwardly factual statements about their products to consumers.
‘Thanks to this regulatory mess, by 2022 we could face the bizarre situation where cigarettes, which kill half of their lifelong users, remain abundant and new cigarettes can come to market with ease but products that are safer and help people quit smoking will be regulated out of existence,’ said Bentley.
‘But this outcome is not inevitable, with the deeming rule’s facing a fresh legal challenge.
PLF’s lawsuit against the FDA argues the deeming rule is unconstitutional and threatens individual liberty.’
Bentley (@gbentley1) is a contributor to the Washington Examiner’s Beltway Confidential blog. He is a consumer freedom research associate at the Reason Foundation and was previously a reporter for the Daily Caller.
His piece, which includes details about the PLF’s key arguments, is at: http://www.washingtonexaminer.com/fda-sued-over-unconstitutional-rule-that-threatens-e-cigarette-businesses/article/2647532.
PMI: review continuing
Philip Morris International has made the point that the ‘careful review’ by the US Food and Drug Administration of the Modified Risk Tobacco Product (MRTP) applications for PMI’s heated tobacco device IQOS is continuing.
On January 24 and 25, the Tobacco Products Scientific Advisory Committee (TPSAC) carried out, at the request of the FDA, an investigation into the scientific issues related to these MRTP applications and broadly recommended that they be rejected.
On a note posted on its website yesterday, PMI said that experts from PMI and Philip Morris USA Inc. had presented to the TPSAC meeting.
‘The meeting was part of the FDA’s review of PMI’s request to commercialize IQOS in the US as a “Modified Risk Tobacco Product”,’ the note said. ‘US law and policy recognize product innovation as important to the 40 million American men and women who smoke.
‘To advise the agency on PMI’s applications, the committee covered a wide range of scientific, technical, and consumer-communications topics. It raised questions and probed the likelihood and magnitude of potential benefits as well as how best to address possible unintended use.
‘Although the Committee did not agree with some of the specific language of proposed risk and harm consumer communications, it confirmed that the evidence supported the statement that switching completely to IQOS significantly reduces exposure to harmful chemicals.’
Meanwhile, PMI’s CEO, André Calantzopoulos (pictured), described the committee’s two-day discussion as a milestone event. “We thank the agency and the committee for their interest in our scientific dossier and our determination to enable adults who smoke to have access to and information about better alternatives,” he was quoted as saying. “I am deeply grateful to my colleagues for their exceptional work on our application to the FDA and for the presentations last week. Our science and the commitment of our people give me confidence that we will realize our vision of a smoke-free future.”
The PMI note said that the company believed the committee’s interactions with presenters and its discussion reflected respect for PMI’s scientific data and commitment to bring IQOS to the US.
‘The Committee delved into such areas as inferences about long-term health outcomes, quantification and comparison of risk, the best way to formulate consumer information, and areas for post-market surveillance,’ the note said. ‘At the end of the meeting, the members of the committee discussed and voted on particular questions on which the agency requested the committee’s views. TPSAC recommendations and votes are not binding on the FDA.’
Calantzopoulos said that as a next step, PMI looked forward to working with the FDA to clarify outstanding points so as to best assist in the agency’s ongoing decision-making process, which inherently entailed a certain degree of scientific uncertainty pre-market. “As the FDA routinely does with regard to the many products it regulates, I believe the agency will ultimately address that uncertainty in the best interest of people who smoke,” he said.
PMI has submitted also a Pre-Market Tobacco Application (PMTA) to the FDA which, if granted, will permit the commercialization of IQOS in the US without modified risk messages. This application was not before the committee because it follows a separate regulatory pathway.
Under agreements with PMI, PM USA is licensed to sell IQOS in the US should PMI receive a PMTA marketing order from the FDA
No safe smoking level
There is no safe level of smoking, according to the findings of a BMJ study reported by Alex Therrien for BBC News.
The BMJ researchers reportedly found that smokers needed to quit cigarettes rather than cut back on them to lower significantly their risk of heart disease and stroke.
People who smoked even one cigarette a day were still about 50 percent more likely to develop heart disease and 30 percent more likely to have a stroke than were people who had never smoked.
There was therefore no safe level of smoking for such diseases, the researchers said.
Their analysis of 141 studies, published in the BMJ, indicates a 20-a-day habit would cause seven heart attacks or strokes in a group of 100 middle-aged people.
If the people in that group cut back to one cigarette a day the result would still be three heart attacks.
Therrien said, however, that ‘an expert’ had pointed out that people who cut down were more likely to stop.
Cardiovascular disease, not cancer, is said to be the greatest mortality risk for smoking, causing about 48 percent of smoking-related premature deaths.
While the percentage of adults in the UK who smoke has been falling, the proportion of people who smoke one to five cigarettes a day has been rising steadily.
Grants-offer spurned
Seventeen public health schools in the US and Canada yesterday pledged to refuse research money from the New York-based Foundation for a Smoke-Free World (FSFW), according to a story by Collin Binkley for WHSV Online relayed by the TMA.
Presumably they have pledged not to apply for such grants.
FSFW was launched in September with a $1 billion grant from Philip Morris International.
The foundation has yet to issue any funding but it has received proposals that are currently under review.
“The idea of taking money that’s from the tobacco industry is just antithetical to everything we do,” said Karen Emmons, dean for academic affairs at Harvard’s public health school.
A letter signed by the 17 college deans said both the tobacco industry and PMI had a long history of funding research in ways meant purposely to confuse the public and advance their own interests.
The foundation has said it will pay for research that helps smokers quit, helps tobacco farmers find other livelihoods and develops reduced-risk alternatives to traditional cigarettes.
Derek Yach, chief of the FSFW and a former executive of the World Health Organization (pictured), said that the foundation was “fully insulated” from industry influence.
Nicotine forum on film
The organizers of the Global Forum on Nicotine say that their fifth annual event, GFN18, will include the first GFN Film Festival.
The festival is being organised in association with Attention Era Media, the makers of ‘A Billion Lives’, which was shown at a previous GFN event.
Aaron Biebert, the director of A Billion Lives, who will curate the festival, said that reversing propaganda and fear tactics would take more than a single movie. “It will take a community of educators, leaders, and influencers who are educated and excited,” he said. “A film festival focused on tobacco harm reduction will make a huge impact by inspiring filmmakers to take up the cause and help get the truth in front of the public. I am delighted to be leading this effort and believe that together we can make a difference.”
The festival will feature films up to 15 minutes long.
The makers of the films chosen for inclusion will be invited to attend GFN18 and the organisers hope to have short Q&A sessions with them following the screenings, which will take place within the conference venue and will be open to everyone attending the conference.
The conference is scheduled to be held at the Marriott Hotel, Warsaw, Poland, on June 14-16.
The festival will take place on June 15 and 16, with each of the entries being eligible for the ‘Best Picture’ award, to be decided by a jury, chaired by Biebert, who will also present the award during the closing session of the GFN.
The winning entry will be shown also during the closing session.
More information about the festival is available at: filmfest@gfn.net.co.