A Danish member of the EU Parliament has asked the Commission whether the requirement for unique, tobacco-product identifiers will be waived in the case of products manufactured in the EU but intended for export to countries that do not allow such markings.
In a preamble to his questions, Bendt Bendtsen said the rules of the Commission Implementing Regulation (EU) 2018/574 of December 15, 2017, on technical standards for the establishment and operation of a traceability system for tobacco products required in Article 6(1) that manufacturers marked each pack produced in the Union with a unique identifier.
‘What does the Commission intend to do in order to ensure that no tobacco products produced in the EU are prevented from being imported into non-EU countries as a result of the new Track and Trace rules, which require all EU-manufactured products to show a mandatory unique identifier?’ he asked.
‘Does the Commission intend to take steps to suspend said requirement for products manufactured for import into countries which, as of 20 May 2019, do not allow imports of tobacco products produced in the Union as a result of the EU requirement of a unique EU identifier?’
The Commission is due to answer in writing.
Category: Packaging

Identifying a problem

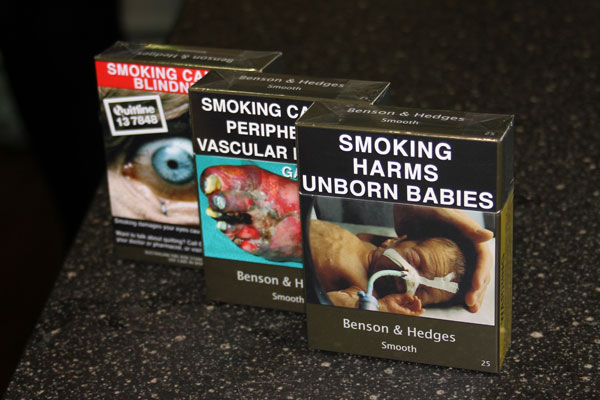
Standardized packs on way
Belgium is to introduce standardized tobacco packaging, according to a story in The Brussels Times quoting the Federal Minister for Public Health Maggie De Block.
The minister, who announced the plan a matter of weeks ago, was said to have received ‘rapid agreement’ from government colleagues.
The story suggested the new packaging would reflect that first introduced in Australia, with huge health warnings and minimal branding beyond the brand name.
No other details were given.
Graphic warnings on order
A US federal court yesterday ordered the Food and Drug Administration (FDA) to issue expeditiously a final rule requiring graphic health warnings on cigarette packs and advertising, as mandated by a 2009 federal law, according to a statement posted on the Campaign for Tobacco-Free Kids website.
The statement was attributed to the American Academy of Pediatrics, the Massachusetts Chapter of the American Academy of Pediatrics, the American Cancer Society, the American Cancer Society Cancer Action Network, the American Heart Association, the American Lung Association, the Campaign for Tobacco-Free Kids and the Truth Initiative
The ruling by district judge Indira Talwani of the district court for the District of Massachusetts was said to have been in response to a lawsuit filed in October 2016 by eight public health and medical groups and several individual pediatricians.
‘Judge Talwani agreed with the health groups that the FDA has both “unlawfully withheld” and “unreasonably delayed” agency action to require the graphic warnings,’ the statement said.
‘Judge Talwani set a deadline of September 26, 2018, for the FDA to “provide to this court an expedited schedule for the completion of outstanding studies, the publication of the proposed graphic warnings rule for public comment, review of public comments, and issuance of final graphic warnings rule in accordance with the Tobacco Control Act.’
The statement described the ruling as a major victory in the fight against tobacco use, which was said to be the nation’s number one cause of preventable death.
‘In accordance with the court’s order, we urge the FDA to quickly issue, finalize and implement a rule requiring graphic cigarette warnings,’ the statement said. ‘The current US cigarette warnings, which are printed on the side of cigarette packs and haven’t been updated since 1984, are stale, unnoticed and a major impediment to greater progress in reducing cigarette smoking.
‘Studies around the world have shown that graphic warnings are most effective at informing consumers about the health risks of smoking, preventing children and other non-smokers from starting to smoke, and motivating smokers to quit.
‘Requiring graphic cigarette warnings in the US will protect kids, save lives and reduce tobacco-related health care costs, which total $170 billion a year.’
Evidence plainly missing
Lithuania’s Prime Minister Saulius Skvernelis has said that there are no compelling arguments in support of the claim that the introduction of standardized tobacco production would cut smoking prevalence, according to a Baltic News Service story relayed by the TMA.
Proposed amendments to Lithuania’s Law on the Control of Tobacco, Tobacco Products and Related Products drafted by the Ministry of Health would require standardized packaging for cigarettes, ban the retail display of tobacco products, and prohibit smoking on residential balconies, in outdoor cafés, at beaches and other outdoor areas.
Skvernelis said that while the Government supported a ban on tobacco product displays in shops, it did not see sufficient arguments for introducing standardized packaging for cigarettes.
He said the proposal should be amended to protect non-smokers’ rights while balancing the interests of smokers, “because imposing bans and restrictions without offering alternatives is a simple way, but it was not approved”.
The introduction of standardized packaging was a highly-debatable measure, he added, and there were no clear arguments that it would cut smoking prevalence.
New warnings in India
The Indian Ministry of Health and Family Welfare has released new images to be included as graphic health warnings on tobacco products starting September 1.
According to a News18.com story relayed by the TMA, last week, the Supreme Court said that tobacco products would continue to carry graphic warnings covering 85 percent of the packaging space.
The government apparently released two separate set of images, the first of which will be used on tobacco products for 12 months beginning September 1, with the second to be used subsequently.
‘All tobacco products manufactured or imported or packaged on or after 1st September 2018 shall display Image-1 and those manufactured or imported or packaged on or after 1st September 2019 shall display Image-2,’ the ministry said in a statement.
‘Any person engaged directly or indirectly in the production, supply, import or distribution of cigarettes or any tobacco products shall ensure that all tobacco product packages shall have the specified health warnings exactly as prescribed.’
Violation of the provision is a punishable offence with imprisonment or fine as prescribed in Section 20 of the Cigarettes and Other Tobacco Products (Prohibition of Advertisement and Regulation of Trade and Commerce, Production, Supply and Distribution) Act, 2003.
Tougher smoking bill
A bill that would toughen anti-smoking legislation in South Africa has been submitted to parliament, according to a story by Nomvelo Chalumbira for Reuters, quoting a health ministry spokesman.
The Control of Tobacco Products and Electronic Delivery Systems Bill would restrict smoking in public places, require standardized tobacco packaging, ban point-of-sale advertising and displays, and scrap the sale of single cigarettes.
Health ministry spokesman Popo Maja was said to have told Reuters that the bill, submitted to parliament for review last week, sought to comply with standards set by the World Health Organization’s (WHO) Framework Convention on Tobacco Control, which South Africa signed in 2005.
“Everything has been taken into consideration,” he was quoted as saying. “What WHO and our country are saying is that it is important for us to make sure that we have a healthy workforce.”
As with previous changes to tobacco legislation in South Africa the bill is facing a backlash from businesses who may suffer from tougher smoking rules. Japan Tobacco International (JTI) was said to have paid for a radio advert aired this month that encouraged the public to protest against the proposals, which were published by the Department of Health in May. “What if your loved one got put in jail because they smoke? It is just one step of the bill controlling your lifestyle choices. Join us in saying #HandOffMyChoices,” said the JTI-funded radio advert broadcast by 702 Talk Radio.
JTI was said not to have respond to several requests for comment about the advert and the #HandOffMyChoices campaign.
The Tobacco Alcohol and Gambling Advisory Advocacy and Action Group (TAG), which campaigns for tobacco control, has said it will make a complaint to regulators about the radio advert, which it believes breaches laws on tobacco advertising.
Warnings on hold
The US Food and Drug Administration has issued a new guidance about how it intends to comply with a recent court order by not enforcing the warning statement requirements for cigars and pipe tobacco products until 60 days after the final decision of the Plaintiffs’ appeal.
In addition, the guidance, Compliance Policy for Certain Labeling and Warning Statement Requirements for Cigars and Pipe Tobacco, states that the agency does not intend to enforce the labeling requirements under sections 903(a)(2) and 920(a) of the Food, Drug, and Cosmetic Act for cigars and pipe tobacco while the injunction remains in effect. During this time, however, cigar and pipe tobacco firms may add the warnings and make these labeling changes.
In a note issued through its Center for Tobacco Products, the FDA said it had revised also the following guidance documents to reflect the compliance policy outlined above:- Submission of Warning Plans for Cigars
- FDA Deems Certain Tobacco Products Subject to FDA Authority, Sales and Distribution Restrictions, and Health Warning Requirements for Packages and Advertisements
- Compliance Policy for Required Warning Statement on Small-Packaged Cigars
- Extension of Certain Tobacco Product Compliance Deadlines Related to the Final Deeming Rule
- Tobacco Retailer Training Programs
The note drew attention also to a new tobacco compliance webinar, Retailer Requirements: New Warning Statement Requirements for Certain Tobacco Products. The webinar is said to address the addictiveness warning statement requirement and which tobacco products it applies to, and to answer some frequently asked questions from tobacco retailers about the warning statement requirements.
The FDA said it had created too several visual examples of the required addictiveness warning statement on different tobacco product packaging, and that it had developed a side-by-side product and warning statement chart.
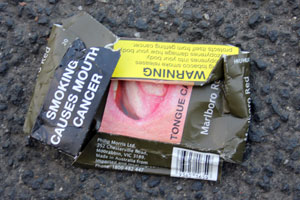
New purulent warnings
India’s Supreme Court yesterday declined to stay the central Government’s new tobacco-packaging rules that require an increase in the size of pictorial health warnings from 40 percent to 85 percent from September 1, according to a story by Bhadra Sinha for the Hindustan Times.
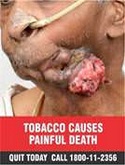
The Cigarettes and other Tobacco Products (Packaging and Labelling) Second Amendment Rules, 2018, require the rotation of graphics and the replacement of current images. The image of a person’s throat with a hole in it, for instance, is to be replaced with more “gruesome pictures of a person’s lips with diseased and purulent growth”.
And in line with this change, the written warning, ‘Smoking causes throat cancer’ is to be replaced with ‘Smoking causes painful death’ and Tobacco causes cancer’, in white upper-case letters against a red background.
The rules require also that packs carry a quit number through which tobacco-product users can connect with online assistance.
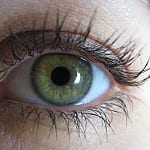
Blind faith in warnings
Reports in Australia based on a study published in the Medical Journal of Australia (MJA) raise some interesting questions about smokers’ thinking; or perhaps about the way that anti-tobacco operatives think that smokers think.
According to a story by Tegan Taylor for the Australian Broadcasting Corporation, the MJA study asked 1,800 Australians about whether they thought smoking increased the risk of 23 conditions shown to be associated with tobacco use, such as lung cancer, stroke and diabetes.
While more than eight in 10 participants knew lung, throat and mouth cancers, heart disease and emphysema were linked to smoking, a much smaller proportion were aware it was associated with erectile dysfunction, female infertility, diabetes and liver cancer.
Dr. Michelle Scollo of Cancer Council Victoria, which ran the study, was quoted as saying the results of the study showed the current warning labels were doing their job, and that it might be time to expand them.
“It was predictable and pleasing that smokers knew about the health effects that have been highlighted in the current sets of warnings and media campaigns,” Scollo said.
But this seemed not to be the case entirely. According to the story, which was illustrated – perhaps ironically – with a huge picture apparently of a Department of Health and Ageing mock-up of a cigarette pack showing a current graphic health warning in which somebody’s eye was being held open with a metal instrument and that bore above the picture the legend: SMOKING CAUSES BLINDNESS, blindness was one of conditions people were least likely to associate with an increased risk caused by smoking.
Scollo went on to say that fewer than half of the people who responded to the study realised smoking could reduce fertility, something that could have a major impact on the course of people’s lives. “There’s a lot that people need to appreciate,” she was quoted as saying.
The current set of graphic warning labels have been in place since 2012 and Scollo hopes the results of the study will lead to an expanded campaign including new graphic warning labels, showing more of smoking’s health risks.
“People need continuous reminders of these sort of things if they’re going to remember them but I don’t see why we need to be limited to just 14 warnings,” she said.
“I think we need as many warnings as we need to adequately warn people about the risks they face.”
WTO appeal likely
An appeal is expected to be made by one or more of the complainants that referred Australia to the World Trade Organization (WTO) over the country’s introduction of plain tobacco packaging, according to Professor Tania Voon of the Melbourne Law School, University of Melbourne.
It has been widely reported, including here, that the WTO recently ruled in favor of Australia.
In a lengthy piece on the Intellectual Property Watch website, Voon said that scholars and professionals in intellectual property, trade and public health were likely to be digesting the 900-odd page WTO Panel Reports in Australia – Tobacco Plain Packaging, circulated on June 28, for some time.
‘An appeal by one or more of the complainants (Cuba, the Dominican Republic, Honduras and Indonesia) is expected, to be launched between 20 and 60 days after circulation, in accordance with the WTO’s Dispute Settlement Understanding (DSU, Art 16),’ she said.
‘Although Australia won the dispute in its entirety, the country could also choose to appeal certain intermediate findings of the Panel, such as its interpretation or application of particular WTO provisions.’
Voon went on to say that, in theory, an appeal would take an additional 90 days, but that the duration of appeals had been significantly higher in recent years, especially in more complex cases. And Australia – Tobacco Plain Packaging was a highly complex case, involving record numbers of third parties (e.g. 38 in the complaint brought by Honduras) and voluminous evidence presented on both sides (e.g. as summarised in the 150-page Appendices to the Panel Report).
Voon’s piece goes on to give details of the Panel’s decisions and the reasons behind those decisions.