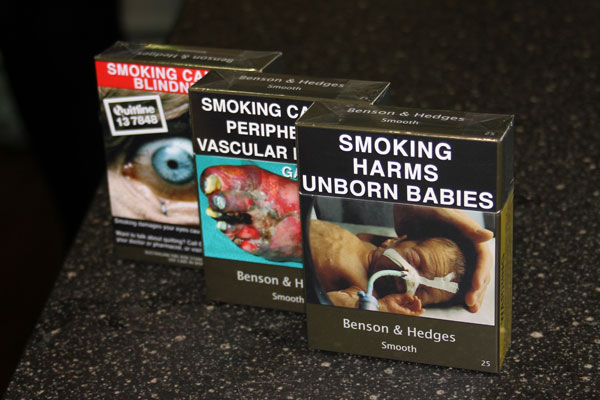
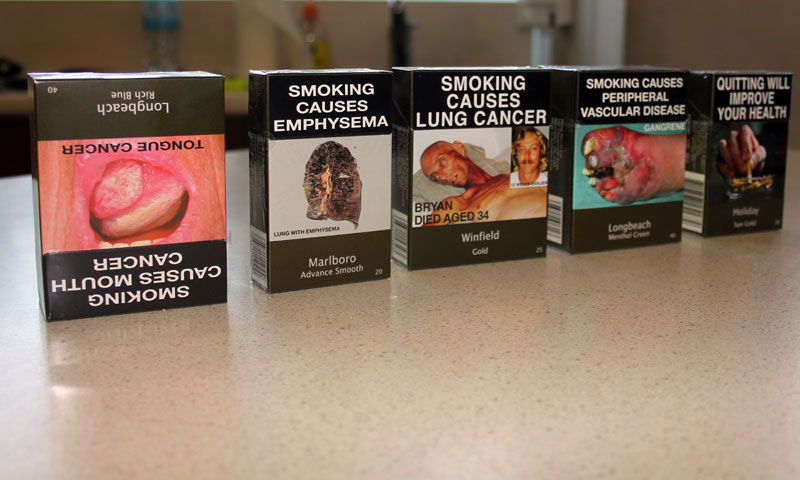
One year on from the introduction of standardized packaging for tobacco in the UK, new research commissioned by Japan Tobacco International and conducted by the independent polling company Kantar TNS, has revealed that the majority of the UK public are not supportive of the policy.
In a note posted on its website JTI said the largest public opinion poll of its kind since standardized tobacco packaging was introduced, research had found that UK citizens were concerned their government had imported a failed policy from Australia without fully evaluating the potential negative consequences.
The poll had found that:
* ‘Almost two-thirds of the UK population believe that plain packaging will not achieve its primary objective of reducing smoking rates (65 percent) and is a poor use of government resources (65 percent).
* ‘Three out of every five UK adults (58 percent) believe plain packaging will lead to an increase in the number of illegal cigarettes sold in the UK.
* ‘If the UK Government had yet to decide on plain packaging and was considering whether to introduce it today, 69 percent of UK adults believe they should either reject the policy (35 percent) or wait for more evidence of its effectiveness from Australia (34 percent).
* ‘72% of respondents believe the Government would either a) fix a policy review/ignore evidence that went against a preferred policy (29 percent), or b) be reluctant to change their preferred policy if the evidence was weighted against it (43 percent).’
Respondents reportedly were critical also of how the Bill became law:
* ’68 percent think the Government changed the decision-making requirements it had previously set out in order to push it through.
* ‘72% think it was important for the Government to research the links between illegal tobacco and terrorist organizations.’
JTI said that, one year on, the latest data showed no impact on tobacco sales or smoking rates in the UK, and that counterfeit standardized packs had been discovered on high streets as early as one month after the implementation of standardized packaging.
‘An analysis conducted by JTI on UK counterfeit samples shows that tar, nicotine and carbon monoxide levels far exceed those allowed in the country,’ the note said. ‘But this is only the tip of the iceberg: in some cases, counterfeits have been found to contain heavy metals such as arsenic, cadmium and lead, along with other toxic contaminants: asbestos, mold, dust, dead flies, rat droppings – and even human excrement.’
“Plain packaging is failing in the UK, as it has in Australia and France, and as we always warned that it would,” states Jonathan Duce, head of external communications at JTI’s global headquarters in Geneva.
“Rather than wait for results to emerge from Australia – as originally committed to by Health Secretary Jeremy Hunt – the government pushed through a policy without waiting for hard evidence or research into the consequences. Plain packaging should never have been introduced in the UK, and other governments considering the measure should think twice before importing this failed experiment.”
Category: Packaging

Plain unpopular

A plain failure of policy
Campaigners have called for an independent ‘root and branch’ review of the impact of all tobacco regulations introduced in the UK since 2010, including that requiring standardized tobacco packaging and a tobacco-products display ban at retail outlets.
The call came after evidence emerged that smoking levels had gone up since the introduction this time last year of standardized packaging and other measures.
‘According to independent research, recent statistics suggest that smoking rates in England were higher than for the same time last year before plain packaging was fully implemented on 20th May 2017,’ a Forest press note said.
‘The figures, published this week by the Tobacco Manufacturers Association, follows evidence that plain packaging has also failed to reduce smoking rates in Australia and France.’
‘In 2012 Australia became the first country in the world to impose standardized packaging on tobacco products. Five years after its implementation, data published by the Australian government showed that the measure had made no significant difference to the daily smoking rate.
‘Instead low-priced cigarettes doubled their market share between 2011 and 2016 (from 29 percent to 60 percent) at the expense of medium- and high-priced cigarettes (from 19 percent to 10 percent) as people switched to cheaper brands.
‘France introduced plain packaging of tobacco products in January 2017. One year later data published by the public authority OFDT showed that the number of cigarettes shipped to retailers remained largely unchanged, with a decrease of just 0.7 percent in 2017.
‘The failure of plain packaging to reduce smoking prevalence in France was acknowledged by health minister Agnès Buzyn during a parliamentary debate. According to the minister, plain packaging “does not lead smokers to quit smoking”. She added that she didn’t know if the introduction of plain packaging in France “has been effective in preventing youth from starting smoking”.
As well as standardized packaging, May 2017 saw the introduction of other tobacco control measures. The European Commission’s revised Tobacco Products Directive forced all EU member states to adopt larger health warnings and prohibit the sale of smaller packs of cigarettes and rolling tobacco. A ban on menthol cigarettes will follow in 2020.
“The experience of Australia, France and Britain suggests that plain packaging doesn’t lead to a decline in smoking rates,” said Simon Clark (pictured), director of Forest, the consumer group.
“Governments blunder on from one tobacco control measure to another, regardless of their impact.
“It’s time for an independent root and branch review of all the tobacco control measures introduced since 2010, including plain packaging and the behind-the-counter display ban.”
The failure of standardized packaging was said to be an indictment of the haste with which the policy was pushed through parliament before the 2015 general election.
“Plain packaging has nothing to do with health,” Clark said. “The decision to introduce it in the UK was based not on evidence that it would reduce smoking rates but on party politics.
“It wasn’t right then and it isn’t right now.”
Opting for quit-or-die
The government of South Korea is changing the health warnings required on packages of combustible cigarettes and heat-not-burn (HNB) products, according to a story by Lee Kyung-min for the Korea Times.
The change will be most pronounced in the case of HNB products, whose packs previously carried an image of what the story described as a needle but that looked more like a syringe. The image was said by many to be unclear and ineffective.
From December, manufacturers of HNB products will be required in include on their packs graphic warnings about health risks associated with smoking, including cancer, similar to the warnings carried by packs of combustible cigarettes.
The Times said the government planned to dispel the idea that HNB products were less harmful [than were combustible cigarettes] and therefore should remain exempt from stringent health policy.
‘HNB-produced smoke contains over 4,000 chemicals, of which more than 70 are carcinogenic substances, known to cause, initiate or exacerbate cancer,’ the Times reported.
‘According to the World Health Organization (WHO), all forms of tobacco use are harmful, including HNBs.’
Yesterday, the Ministry of Health and Welfare unveiled a set of new graphic warnings with more ‘disturbing’ messages than those currently used.
The new warnings must replace the current ones by December 23.
The introduction of the new measures followed what was described as ‘a one-year in-depth deliberation commissioned by a 13-member special committee comprised of government officials and private experts’. A survey of 1,500 smokers and non-smokers was said to have been conducted to reflect public opinion.
According to data from the Ministry of Strategy and Finance, HNB products accounted for eight percent of the South Korean cigarette market in January, up from three percent seven months earlier.
Plain packaging failing
The UK-based Tobacco Manufacturers’ Association (TMA) yesterday published data showing how standardized tobacco packaging is failing in the UK.
The TMA said that while reducing smoking levels had been a key objective behind the introduction of standardized packaging in May 2017, the evidence indicated that there had been an upturn in smoking levels in recent months.
‘The Smoking Toolkit Study has found that on a three-month rolling average, from December 2017 to March 2018, smoking rates in England were higher than for the same time last year before plain packaging was fully introduced,’ the TMA said in a press note.
‘The TMA estimates that if the same effect was seen across the UK, there would be approximately 350,000 more adult smokers in March 2018 than a year before plain packaging was fully introduced.
‘This reflects international evidence that plain packaging doesn’t deter smoking. France introduced plain packaging in January 2016. The French Health Minister, Agnes Buzyn, recently admitted that: “Plain packaging did not contribute to the decrease of official tobacco sales”.’
At the same time, the TMA said, standardized tobacco packs had delivered a huge benefit to criminals because counterfeiting a single pack design was easier, cheaper and more profitable than was counterfeiting different designs.
‘Counterfeit plain packaged tobacco products have already been found across the country,’ the TMA said.
‘The Government was warned this would happen by the tobacco industry, law enforcement and intellectual property experts.’
Meanwhile, the TMA said that new polling it had conducted during the past two months had found that smokers were being pushed towards the illegal market by plain packaging. Over a quarter of UK smokers said that the measure had made them more likely to buy untaxed tobacco, the equivalent of 1.9 million people across the UK.
“The recent evidence shows that plain packaging appears to be failing in the UK like everywhere it has been introduced,” said Giles Roca, director general of the TMA.
“It appears not to be delivering the health outcomes it was claimed it would bring while at the same time is proving to be a boon to the black market by encouraging smokers to buy from illicit sources.
“The Government should recognise that plain packaging is failing and undertake a full and immediate review of this policy.”
Appeal package
Ujal Singh Bhatia, chairman of the World Trade Organization’s appellate body, has said he expects Cuba, the Dominican Republic, Honduras and Indonesia to appeal against the WTO’s yet-to-be-publicized ruling on Australia’s law on standardized tobacco packaging, according to a Bloomberg story relayed by the TMA.
The ruling will apparently say that the law was a legitimate public health measure and did not constitute an illegal barrier to trade.
Bhatia said once an appeal was triggered, the WTO appellate body was required to consider the matter and issue a ruling within 90 days.
However, he added that the timeline might be extended because of the complexity of the dispute and a shortage of panelists in the WTO’s appellate system.
The countries alleged that the measure imposed ‘unfair’ restrictions on the use of trademarks, geographical indications and other markings in violation of several WTO agreements.
The WTO has yet to circulate publicly its final dispute ruling, partly because of the time required to translate the ruling.
Improving the TPD
Enforcing basic safety and quality standards within the EU was critical in order to protect consumers and create a level playing field for vaping products, according to Yasuhiro Nakajima, vice president, reduced-risk products at Japan Tobacco International.
Wring a Thought Leader in the Parliament Magazine yesterday, Nakajima said the EU’s revised Tobacco Products Directive (TPD) had created a common framework that had provided the flexibility for vaping to flourish in those member states that were comfortable with it while maintaining safeguards demanded by other countries.
Yet the TPD was not perfect, he said. For instance, relying just on the TPD’s self-notification system was a high-risk approach to ensuring quality.
‘Enforcing basic safety and quality standards is critical in order to protect consumers and create a level playing field for vaping products,’ he said. ‘To not do so would only benefit the unscrupulous.’
The notification problem, Nakajima said, had been compounded by another unintended consequence of TPD rules on e-cigarettes: the fast-growing short-fill e-liquid market. Consumers wanted bigger refill bottles than the TPD allowed. ‘The result is them topping up unregulated non-nicotine bottles with regulated nicotine shots,’ he said. ‘Do health officials know what is in those non-nicotine liquids? No – and this needs to change.’
A final challenge was consumer ignorance on the scientific consensus on vaping’s reduced-risk potential, he said. Public health officials were concerned about the growing gap between what scientists say and what the public believes.
‘The easy solution would be for the European Commission to pass the problem onto manufacturers,’ he said. ‘We could help close the gap if we were allowed to communicate science-based messages to adult consumers.
‘Amendments to the EU rule book should be built on the expert view that vaping products are very different to combustible ones and therefore warrant an entirely different, and more liberal, consumer communication framework.’
Corrective statements online
The US’ major cigarette companies will have to post court-ordered ‘corrective statements’ on their websites from next month.
A note posted on the Website of the US Department of Justice said that on Tuesday the District Court for the District of Columbia had entered a consent order requiring the country’s major cigarette companies to begin posting ‘corrective statements’ on their websites starting on June 18.
‘The order, part of a long-running lawsuit against the cigarette companies, also requires them to attach the same statements to cigarette packages for two weeks at a time, for a total of twelve weeks over two years,’ the note said. ‘The order will also apply to any social media campaigns by the companies to promote cigarettes.
‘The statements address the effects of cigarette smoking and the fact that cigarettes are deliberately designed to create and sustain addiction. As a result of a previous court order, the statements are currently running on television five times per week, and previously ran as full-page ads in about fifty newspapers across the country.
‘The statements specifically state, among other things:- ‘That smoking cigarettes causes numerous diseases and on average 1,200 American deaths every day;
- ‘That the nicotine in cigarettes is highly addictive and that cigarettes have been designed to create and sustain addiction;
- ‘That so-called light, low-tar, and natural cigarettes are just as harmful as regular cigarettes; and
- ‘That second-hand smoke causes disease and death in people who do not smoke.
‘The corrective statements were ordered as part of a 2006 permanent injunction against cigarette companies, including Altria, its Philip Morris USA subsidiary, and R.J. Reynolds Tobacco, to “prevent and restrain” further deception of the American people regarding tobacco use. The order also applies to ITG Brands, which purchased Winston, Kool, and other cigarettes brands from companies in the case.’

Youngsters targeted
The US Food and Drug Administration and the Federal Trade Commission (FTC) have issued 13 warning letters to manufacturers, distributors and retailers about selling e-liquids in packaging likely to appeal to young people.
In a note issued through the FDA’s Center for Tobacco Products, the agencies said that they were targeting those involved in e-liquids used in e-cigarettes with labeling and/or advertising that caused them to resemble ‘kid-friendly food products, such as juice boxes, candy or cookies, some of them with cartoon-like imagery’.
Several of the companies that received warning letters were also cited for selling such products to minors.
“No child should be using any tobacco product, and no tobacco products should be marketed in a way that endangers kids – especially by using imagery that misleads them into thinking the products are things they’d eat or drink,” said FDA Commissioner Scott Gottlieb, M.D, as part of the note.
“Looking at these side-to-side comparisons is alarming. It is easy to see how a child could confuse these e-liquid products for something they believe they’ve consumed before – like a juice box.
“These are preventable accidents that have the potential to result in serious harm or even death. “Companies selling these products have a responsibility to ensure they aren’t putting children in harm’s way or enticing youth use, and we’ll continue to take action against those who sell tobacco products to youth and market products in this egregious fashion.”
Warnings not implemented
More than two years after the government in Nepal decided that tobacco-package health warnings should be increased in size, the requirement has not been implemented effectively, according to a story in The Himalayan Times.
In part, at least, this has to do with the fact that the requirement is being challenged in the Supreme Court.
A 2011 law requiring that health warnings covered 75 percent of tobacco packaging was amended 25 months ago to lift the coverage to 90 percent.
Dr. Tara Singh Bam, the deputy regional director at the International Union against Tuberculosis and Lung Diseases, said that, as per the law, pictorial warnings should be included on the front, back, top, and both sides of every tobacco package. There were five pictures and corresponding text warnings that should be rotated every six months, he said.
Bam added that some tobacco companies were not abiding by the requirements of the law, and he urged the government to take action against those companies.
Dr. Pushpa Chaudhary, secretary at the Ministry of Health, said the responsibility of the Ministry was to draft the bill; it did not have the right to take action against companies not abiding by the law.
But Narayan Prasad Bhandari, director at the Inland Revenue Department, said the Department had already warned the companies of stringent action if they failed to abide by the law.
However, a complicating factor is that a tobacco company is challenging the government’s new law in the Supreme Court, and whereas the hearing had been scheduled for April 18, it has been delayed.
“The Supreme Court has issued a stay order in the case, so we haven’t taken any action against the companies,” said Bhandari. “The issue of revenue collection was also one of the reasons for not taking action against them.”
More plain packaging
Sri Lanka’s Cabinet has approved a proposal for the imposition of standardized tobacco packaging, according to a story by Manjari Peiris for the Asian Tribune.
The proposal was said to have been submitted by the Minister of Health, Dr. Rajitha Senaratne.
The story said that standardized packaging required the removal of all branding, including colors, imagery, corporate logos and trademarks.
Brand names were printed in a specified size and font, and in a specified position on the pack.
Otherwise, packs were presented in a standard, specified color, and included health warnings, mandated information and tax stamps.
It wasn’t clear whether the above specifications were the ones proposed by the health minister and accepted by the Cabinet; or whether they were simply those generally used in countries where such packaging has been introduced already.