The industry tried to comply as best as possible; many premium e-liquid products that had previously been in glass bottles were switched to plastic bottles because there were no flow restriction capabilities in glass bottles. Glass bottles typically use droppers, whereas plastic bottles allow for squeezing. However, one innovative company in this space, Fluid Certify, has developed patent-pending technology to help address the glass bottle flow restriction issue.
Fluid Certify was founded by Cole McDonald, who also founded McDonald Vapor Co., an e-liquid manufacturer. In 2020, Cole passed away in a tragic climbing accident, and his mother, Lola McDonald, has kept the business going. Before Cole’s passing, he created flow restriction technology for glass bottles. Cole’s e-liquid products were packaged in unique glass bottles, and when the CPSC came out with its guidance, he conceived of his patent-pending technology to allow himself and others to maintain a level of excellence and offer alternatives to more generic plastic bottles.
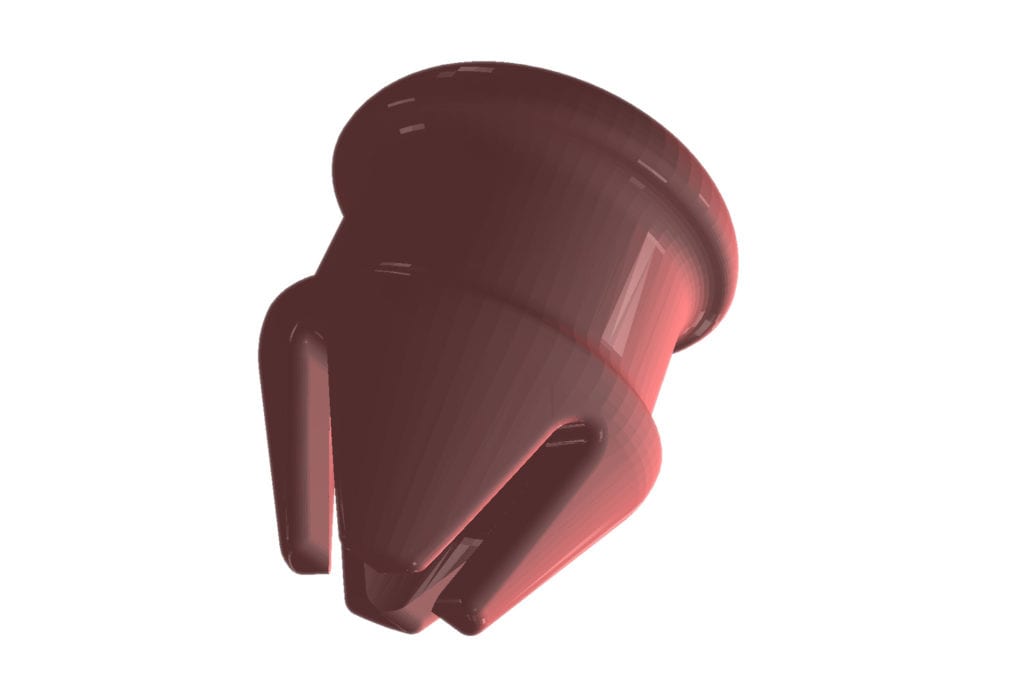
The material used in Fluid Certify’s glass bottle flow restrictors is FDA food grade and offers flexibility and strength. It uses vacuum pressure and gravity to allow for repeated, uninterrupted use of a pipette. While the company is not currently mass producing the technology, it is not out of the question, according to Lola.
“Cole had a passion for the industry … he had no excuses for failure or blame—he found solutions,” Lola said of her son. His creativity and inclination for inventing opened the door to potentially “change things for the better” as he hoped to do. The company’s website, www.fluid-dynamic.com, offers more information on how to access the technology.
So what about the labels? While e-liquid labels are required to have basic information (name and place of business, amount of liquid contained, a nicotine addiction warning, etc.), the most critical FDA requirement is that they not appeal to youth. This is a broad requirement, and many companies received warning letters for products that mimicked kids’ foods, such as candy and juice. “My advice to clients is to keep their labels as simple as possible, keep it as mature as possible, and don’t use too many colors or graphics kids would enjoy,” said Chowdhury. The industry is “very subjective and competitive,” he added.
E-liquid packaging requirements are many, though it takes some digging to find them and guidance to navigate the nuance. With the growth and change in this industry, it will be interesting to see what comes next for e-liquid bottles and packaging.