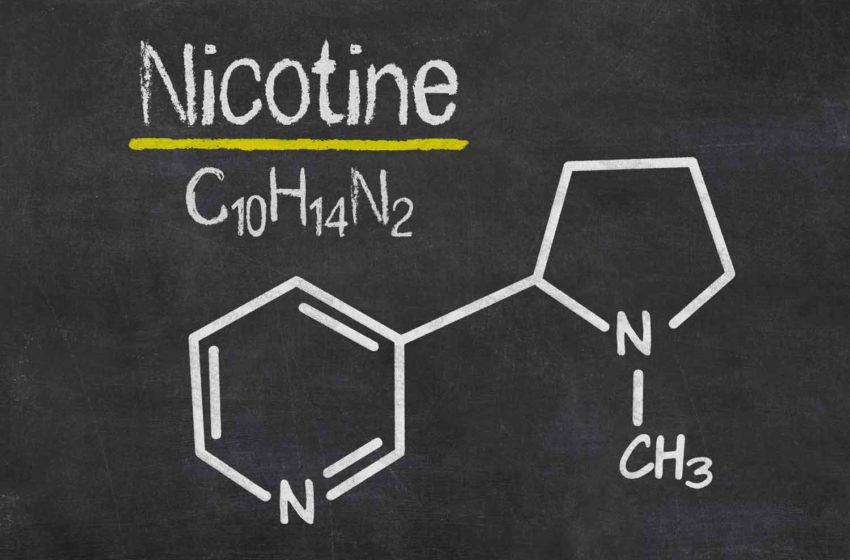
Next Generation Labs, which manufactures and markets bulk R-S, R- and S-isomer synthetic nicotine under its TFN brand, has terminated its Sept. 28, 2020, patent contribution agreement with Kaival Brands Innovation Group and Kaival Labs.
The Patent Contribution Agreement related to the acquisition and commercial exploitation by Kaival of Next Generation Labs’ IP portfolio on combinational use of synthetic R- and S-isomer nicotine ratios in tobacco cessation products.
In a press note, Next Generation Labs said Kaival Labs had published inaccurate and misleading statements relating to the use of patented synthetic nicotine for tobacco cessation products on its website. “Next Generation Labs wants to clarify that as a consequence of Kaival’s failure to perform its obligations under the Patent Contribution Agreement, all rights to the R-S synthetic nicotine cessation patent portfolio fully reverted to Next Generation Labs in May 2021,” Next Generation Labs wrote.
Additionally, Kaival executed a confirmatory transfer agreement with Next Generation Labs relinquishing all rights to the Next Generation Labs patent portfolio for the commercialization of combinational TFN R-S nicotine relating to tobacco cessation products. Kaival is not an authorized agent of Next Generation Labs nor is it authorized to use Next Generation Labs’ name, intellectual property or its TFN trademark on its products, in its product marketing or in any financial prospectus to investors, according to Next Generation Labs.