The smokers’ group Forest says that new tobacco regulations coming into force in the UK ‘infantilise’ consumers and will make no difference to public health.
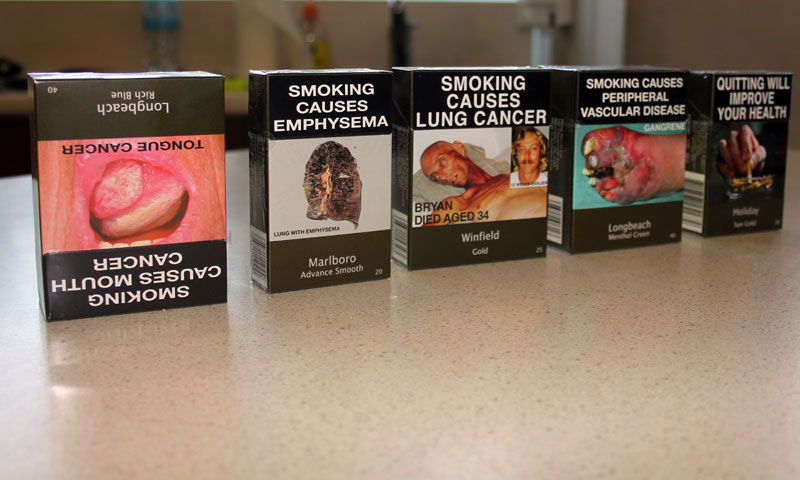
The new rules, which must be fully implemented by this weekend, include a minimum pack size of 20 cigarettes, a minimum pouch size of 30g of rolling tobacco, a ban on branded packaging for cigarettes and rolling tobacco, and the imposition of larger health warnings.
“The new regulations treat adults like naughty children, said Simon Clark, the director of Forest, which campaigns for smokers’ rights. “They infantilise consumers by attacking freedom of choice and personal responsibility.
“Adults and even teenagers are under no illusions about the health risks of smoking. Consumers don’t need larger health warnings to tell them what they already know.
“Banning smaller packs is a pathetic attempt to target the less well-off in the hope they will be forced to quit, but smokers will soon adapt and buy the larger packs instead.
“If you’re trying to cut down it will be harder now because the option of buying a smaller number of cigarettes has been taken away.”
Clark was scathing also about standardized packaging.
He described as absurd the idea that people smoked because of the packaging and said there was no evidence that plain packaging had any impact on youth smoking rates, and without such evidence there was no justification for the policy.
“The new regulations are a disgraceful attempt to denormalize both the product and legitimate consumers,” Clark said.
“There’s no evidence they will have the slightest impact on public health.
“Politicians and tobacco control campaigners are grasping at straws if they think people will give up something they enjoy just because the packaging has changed.”
Clark said the new government [the UK is holding a general election next month] should review the measures as soon as the UK has left the European Union.
“With the exception of plain packaging all these regulations were imposed on the UK by the EU’s Tobacco Products Directive,” he said.
“Brexit will give the government the chance to review the impact of these policies and, where necessary, amend or repeal regulations that deliberately discriminate against millions of adult consumers.”