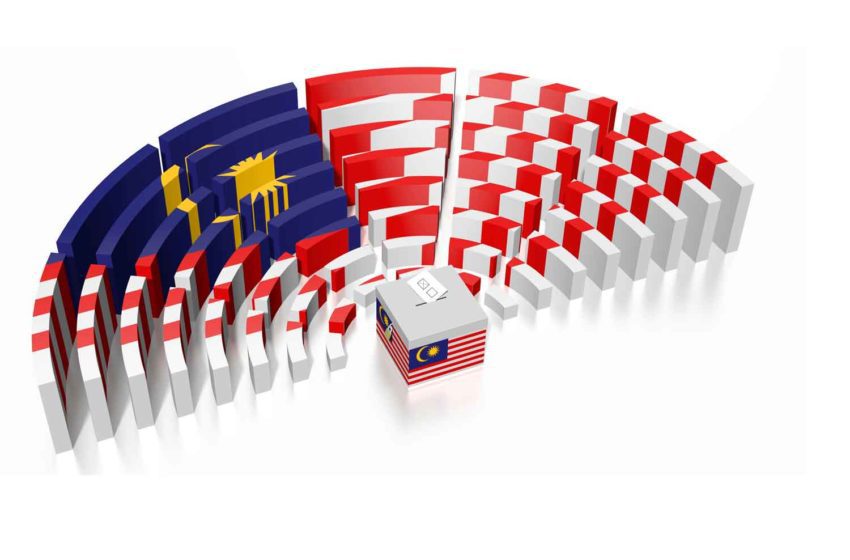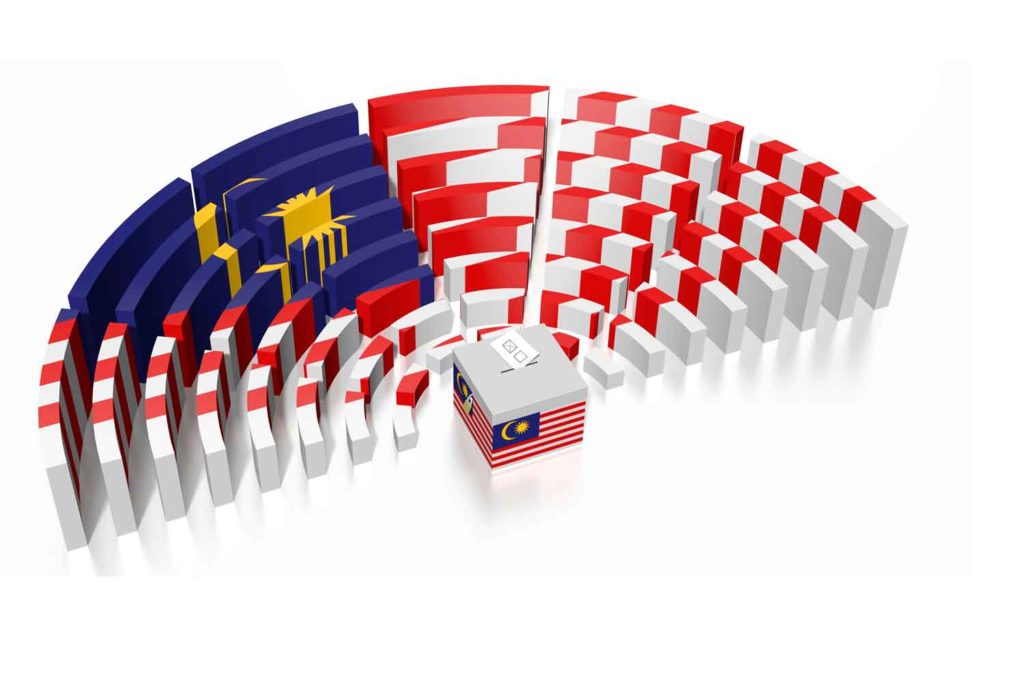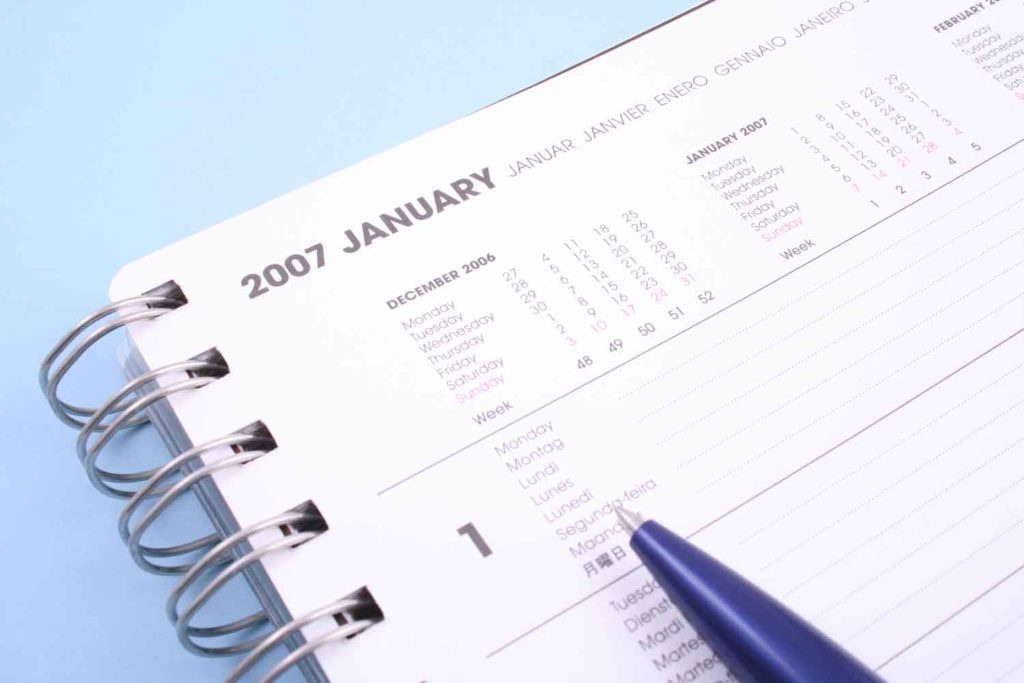
After considering the views of stakeholders, the government of Malaysia has pushed the year limit of its tobacco generational endgame law to 2007 from 2005, reports New Straits Times.
Earlier this year, Health Minister Khairy Jamaluddin tabled a new Tobacco and Smoking Control bill to replace the current tobacco product control legislation under the Food Act 1983. Modeled on similar legislation in New Zealand, the proposal included a provision to ban smoking and prohibit the ownership of tobacco and vape products by those born after 2005.
Postponing the year limit will allow more time for community education, a robust implementation plan and to ramp up enforcement, according to Khairy.
The health minister has been pushing for the Tobacco and Smoking Control Bill in line with efforts to make Malaysia a tobacco-free country by 2040.
He said cigarette smoking would cost the government MYR8 billion ($1.8 billion) to treat lung cancer, heart problems and chronic obstructive pulmonary disease by 2030.
The cabinet gave the green light for the bill on July 14, and it will be tabled and put to a vote in the Parliament’s Lower House this week.
BAT Malaysia said the proposed generational smoking ban is a prohibitive way to reduce the health impact of smoking and will only fuel the illicit tobacco market, which already accounts for almost 60 percent of tobacco sold in Malaysia.
“It has never been tested in the real world, lacks any scientific evidence of effectiveness and is likely to be detrimental to our country’s health agenda,” BAT Malaysia Managing Director Nedal Salem was quoted as saying by The Edge Markets.
He said the Ministry of Health (MOH) should pursue a science-based regulatory framework, informed by the positions of countries such as the U.K., where vaping is acknowledged as significantly less harmful and a viable alternative to reduce smoking prevalence.
BAT Malaysia called on the MOH to include industry players in the overall consultation process in developing appropriate regulations for vapor products.













 “JWEI has been a leader in this industry from the start and this milestone again reiterates our commitment to the industry and public health: ensuring our adult customers continued access to less harmful alternatives to traditional tobacco products, while setting a new standard preventing underage youth access.” said VP of JWEI Group Jason Yao.
“JWEI has been a leader in this industry from the start and this milestone again reiterates our commitment to the industry and public health: ensuring our adult customers continued access to less harmful alternatives to traditional tobacco products, while setting a new standard preventing underage youth access.” said VP of JWEI Group Jason Yao.