U.S. States are failing to invest proceeds from the 1998 Tobacco Master Settlement Agreement (MSA) into tobacco prevention funds, according to a new report from the Campaign for Tobacco-Free Kids (CTFK), American Cancer Society Cancer Action Network, American Heart Association, American Lung Association, Americans for Nonsmokers’ Rights, Robert Wood Johnson Foundation and Truth Initiative shows
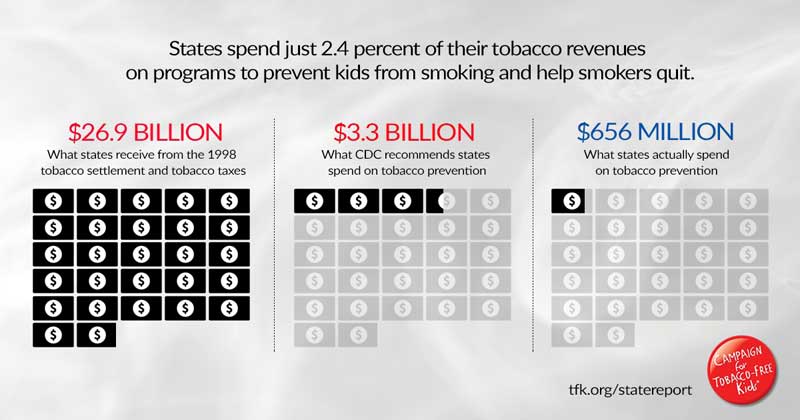
“This year (fiscal year 2021), the states will collect $26.9 billion from the 1998 tobacco settlement and tobacco taxes,” the CTFK wrote in a statement. “But they will spend a paltry 2.4 percent—just $656 million—on tobacco prevention and cessation programs. The total is an 11 percent decrease from last year and less than a fifth (19.8 percent) of the total funding recommended by the Centers for Disease Control and Prevention (CDC).”
Alaska (89.7 percent), Maine (87.4 percent) and Utah (79.4 percent) are the only states to provide at least three-quarters of the CDC-recommended funding for tobacco prevention and cessation programs.
Tobacco companies spend more than $13 to market tobacco products for every $1 the states invest to reduce tobacco use, according to the CTFK. According to the most recent data from the Federal Trade Commission (for 2018), the major cigarette and smokeless tobacco companies spend $9.1 billion a year on marketing.