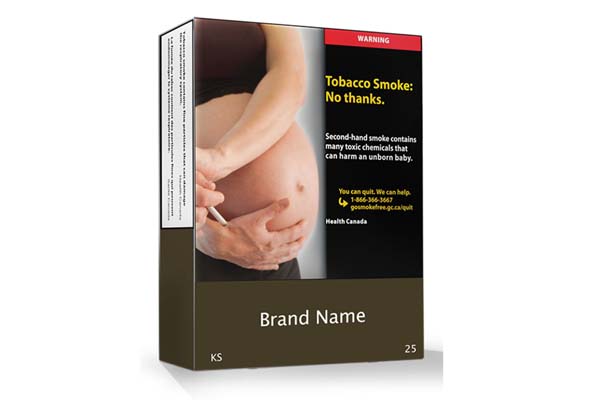
Canada’s plain packaging law will take effect on Nov. 9.
All packaging will feature the same brown base color, basic gray text and minimalist layout under the new requirements. The measure will also standardize the size and appearance of cigarettes, cigars and other products inside the packages.
Tobacco companies will have 90 days to offload their branded inventory.
In 2021, slide-and-shell packages will become mandatory in Canada, providing a wider surface area that will display the largest health warnings in the world, he said.
“The package designs [are] really amazingly glitzy and very attractive, especially to kids,” said University of Waterloo psychology professor Geoffrey Fong, the founder and chief principal investigator of the International Tobacco Control Policy Evaluation Project.
“What we’ve found is that plain packaging has tremendous effects on reducing the appeal of these deadly products.”
Eric Gagnon, head of regulatory affairs at Imperial Tobacco Canada, said plain packaging would boost the illicit sale of tobacco products while disrupting the tobacco supply chain.
“It’s not like you just turn a key on and off,” he said. “You need to change all your artwork, all your equipment, retool all your machines, so obviously, it’s very costly and a very complex operation.”