The commissioner of the US Food and Drug Administration Scott Gottlieb has said that the agency is considering whether it could bring electronic cigarettes into the over-the-counter (OTC) regulatory pathway, according to a CNBC report relayed by the TMA.
Gottlieb said the OTC pathway would give the agency more tools to look at both safety and benefit, study whether or not an e-cigarette promoted smoking cessation, and study toxicity and the way it affects the lung.
This move was said to be part of the sweeping plan to overhaul tobacco regulation that the Commissioner announced in July.
“At the very time I am trying to take nicotine out of combustible tobacco, I don’t want to be sweeping the market of products that provide an alternative to smokers who want to get access to nicotine,” Gottlieb said.
The idea flows from the FDA’s continuum of risk policy that recognizes that conventional cigarettes are the most harmful and other nicotine products are potentially less risky.
Category: Regulation

FDA moving on e-cigs

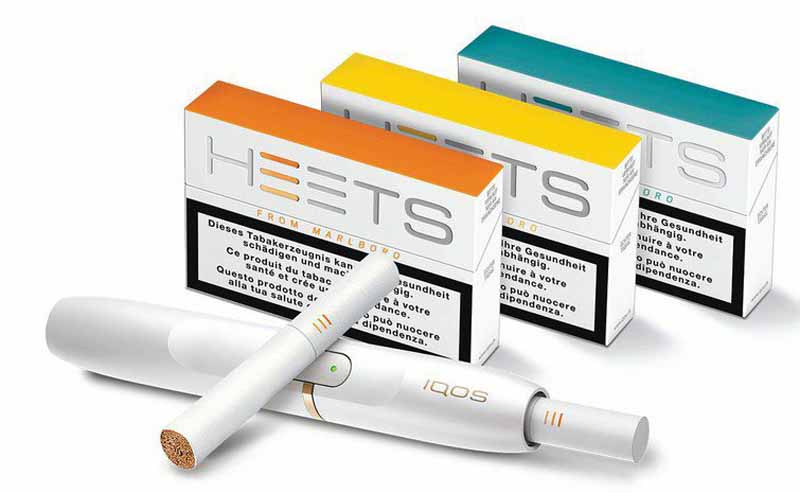
HEETS-ban case dismissed
A Ministry of Health claim that a heated-tobacco product was banned under New Zealand law has been dismissed by a judge sitting alone in the District Court of Wellington.
The case, brought against Philip Morris New Zealand (PMNZ) in respect of HEETS, the consumable item of PM’s IQOS system, claimed that the product was banned under the Smoke-Free Environment Act, which bans tobacco products used for chewing or any other oral use (other than smoking).
But Judge Patrick Butler found that HEETS were not caught within the ambit of s 29 (2) of the Act and dismissed the charge.
While welcoming the decision, PMNZ’s general manager Jason Erickson said the case highlighted the need for urgent reform of regulations surrounding e-cigarettes and other smokeless tobacco products.
“To achieve the Smokefree 2025 goal, men and women who smoke in New Zealand need freely available information and access to a range of better alternatives to cigarettes, including nicotine-containing electronic cigarettes and smokeless tobacco products such as HEETS,” he said.
Vapor lumped with smoke
Greece’s Council of State has ruled that vaping and electronic cigarettes are covered by the laws that restrict smoking and traditional cigarettes.
The ruling by the country’s Supreme Administrative Court means that e-cigarettes cannot be used in public places or on public transport, and cannot be promoted in advertisements.
In reaching its decision, the Court rejected a petition by the Association of Greek E-cigarette Businesses.
But the court added that the Association, which claimed it had been financially harmed by the law, imposed in 2017, may file a lawsuit for compensation.
Plain packaging attacked
The removal of brands from packaging is a ‘gross violation’ of intellectual property rights and has failed to achieve its intended goals, the Property Rights Alliance argues in a letter to the World Health Organization.
According to a story by Claire Stam for EURACTIV.com; in an open letter to WHO director-general Tedros Adhanom Gehbreyesus, an international alliance of 62 think tanks, advocacy groups and civil-society organisations said it was time to end ‘ineffective’ standardized packaging for any kind of product.
The alliance said they had sent the letter in response to a growing number of standardized-packaging tobacco-control measures in a number of countries.
‘Intellectual property rights are human rights enshrined in the Universal Declaration of Human Rights: Article 17, the right to ownership, Article 19, the right to freedom of expression, and article 27, the right to protection of material interests,’ the letter reportedly said.
‘In this regard, even if plain packaging is effective, it should still be repealed, as rights are inalienable and should not be discarded for political purposes.’
Meanwhile, Stam said that, in the eyes of the WHO and public health NGOs, standardized packaging was a key tool to reduce the appeal of smoking, especially among young people.
The full story is at: https://www.euractiv.com/section/health-consumers/news/who-urged-to-end-ineffective-tobacco-plain-packaging/.
Prohibition Lite on the way
In a piece on the Reason website, J.D. Tuccille posits that there’s no particular reason to think that smokers will be happier with denatured tobacco than drinkers have been with weak beer.
In examining the US Food and Drug Administration’s proposal to lower nicotine in cigarettes to minimally or non-addictive levels, Tuccille says that low-nicotine cigarettes sound a lot like the 3.2 percent beer that plagued much of the country after Prohibition. ‘Nobody was happy with the diluted swill, and they tolerated it only if they couldn’t smuggle in something better,’ he writes. ‘Most places have since dumped it, indicating that a taste for the unadulterated product remained strong even after years of restrictions.’
Tuccille’s piece ends by saying that FDA Commissioner Scott Gottlieb’s proposal to mandate low-nicotine cigarettes looks a lot like other well-intentioned but presumptuous efforts to substitute the will of regulators for the desires of the public – Prohibition Lite. And like all such efforts, he says, it’s likely to get people turning up their noses and looking for something better.
Tuccille’s piece is at: https://reason.com/archives/2018/03/26/fdas-low-nicotine-cigarette-scheme-is-an.
FDA 'messing' with cigars
The US Food and Drug Administration has issued an advance notice of proposed rulemaking (ANPRM) seeking comments and scientific data related to the patterns of use and resulting public health impacts of ‘premium’ cigars.
In a note issued through its Center for Tobacco Products (CTP), the agency said it was specifically requesting comments and information not previously submitted in response to its proposed deeming rule.
Submissions, it is said, will be used to inform the agency’s regulatory policies.
The note said that, in 2016, the FDA had finalized the deeming rule extending its authority under the Family Smoking Prevention and Tobacco Control Act to tobacco products not already under its authority, including e-cigarettes, cigars, pipe tobacco and hookah tobacco, among others. The rule included provisions making the sale of these products subject to a range of requirements, such as mandatory age and photo-ID checks to prevent illegal sales to minors; warning statements; submission of health documents; and the prohibition on marketing modified risk products without an FDA order in effect.
‘When proposed, the deeming rule put forth two, alternative regulatory options, one of which would have excluded so-called “premium” cigars from FDA regulation,’ the note said. ‘After carefully considering the public comments on the rule, FDA concluded all cigars pose serious negative health risks, and the evidence did not provide a public health basis for FDA to exclude any type of cigars from regulation.’
Mitch Zeller, director of the FDA’s CTP, said all tobacco products, including ‘premium’ cigars, remained subject to manufacturing, distribution and marketing oversight by FDA.
“However, given the ongoing interest from many parties on this issue ‒ as well as the potential for new data on the topic since the deeming rule published ‒ we’re providing stakeholders an opportunity to submit relevant new information that could inform FDA’s regulation of ‘premium’ cigars.”
Through the premium cigars ANPRM, the agency is requesting comments, data, research results, or other information not submitted in response to the proposed deeming rule on topics including, but not limited to:- ‘The definition of ‘premium’ cigars;
- Use patterns of ‘premium’ cigars generally and among youth and young adults specifically;
- Public health considerations associated with ‘premium’ cigars, including the health effects;
- Studies or information regarding consumer perceptions of the health risks of ‘premium’ cigars; and
- Studies or information on whether any applicable manufacturing, marketing, sale, distribution, advertising, labeling, and/or packaging requirements and restrictions should be applied differently to ‘premium’ cigars compared to other tobacco products, including other cigars.’
The ANPRM will be available for comment from March 26 through June 25.
The ANPRM is one of three previously announced in July 2017 as part of FDA’s comprehensive plan on nicotine and tobacco regulation,’ the note said. ‘Earlier this month, FDA published the other two ANPRMs, “Tobacco Product Standard for Nicotine Level of Combusted Cigarettes” and “Regulation of Flavors in Tobacco Products”.
‘The comprehensive plan serves as a multi-year roadmap to better protect kids and significantly reduce tobacco-related disease and death.’
Ban voted down
Austria’s lower house of parliament voted yesterday to scrap an impending ban on smoking in bars and restaurants, according to a Reuters story.
The vote was a win for the coalition government and came despite opposition from health campaigners and opposition parties.
The ban, which is due to come into effect in May, would bring Austria more into line with fellow EU countries, many of which have stricter smoke-free legislation.
At present, large restaurants in Austria are required to provide separate smoking and non-smoking areas, but the rules are reportedly not rigidly implemented. Smaller restaurants need not have a separate area if the owner agrees to allow smoking on the premises.
More than half a million people in Austria have signed an official petition calling for the ban to go ahead, embarrassing the ruling coalition of conservatives and the far-right Freedom Party (FPO), which has championed both the freedom to smoke and direct democracy.
An FPO demand to have the ban scrapped was written into the coalition agreement struck three months ago.
Now that parliament has approved the bill, it has to be passed by the upper house and signed by the president. It is widely expected to pass in the upper house and to be signed into law.
In a statement, the organizers of the petition, the Vienna’s doctors’ association and the country’s main anti-cancer organization, called the vote a uniquely bad example.
The FPO says a smoking ban would be an unnecessary intrusion on individual liberty and an unfair imposition on bar and restaurant owners.
Opponents say public health is more important and point to the cancer risk posed by passive smoking.
Samoa tables new bill
The government of Samoa yesterday tabled the Tobacco Control Amendment Bill 2018 through which it is proposing, among other things, to regulate tobacco licensing fees imposed on manufacturers, importers, distributors, nightclub owners and hotels, according to a story in The Samoa Observer.
The wide-ranging Bill was introduced in parliament by the Minister of Health, Tuitama Dr. Leao Tuitama.
The bill proposes that people under the age of 21 should be prohibited from selling tobacco products.
It seeks to take control of determining the permitted weight and size of cigarettes.
And it would allow the Ministry of Health to conduct tests on tobacco products without firstly warning manufacturers and importers, and at the cost of the manufacturer or importer.
The Bill seeks also to provide a new system under which manufacturers, importers and distributors of tobacco products would have to be licensed in order to distribute tobacco products.
A flavor of things to come
The US’ Food and Drug Administration has issued an advance notice of proposed rulemaking (ANPRM) in respect of tobacco-product flavors.
In a ‘Special Announcement’ issued through its Center for Tobacco Products, the FDA said it was seeking ‘comments, data, research results, or other information related to the role that flavors, including menthol, play in tobacco product use and potential regulatory options the agency could take, such as tobacco product standards and measures related to the sale and distribution of flavored tobacco products’.
‘This notice, along with another ANPRM issued last week related to reducing nicotine in cigarettes to minimally addictive or non-addictive levels, is part of the FDA’s comprehensive plan on tobacco and nicotine regulation, as announced in July 2017.
‘FDA values the public’s input through the comment process and will consider all submissions as it considers possible regulatory actions on these topics. If FDA decides to issue a rule, the first step would be to issue a notice of proposed rulemaking in the Federal Register, which would give the public another opportunity to comment on the proposal.’
The ANPRM on the role of flavors in tobacco products was made available for comment for 90 days from yesterday.
Meanwhile, the FDA Commissioner Scott Gottlieb, MD, issued a 1,400-word statement on the flavors ANPRM in which he said, in part, that, ultimately, the FDA was working to ensure its policies achieved the greatest public health benefit.
‘As such, we’re proceeding with the utmost caution by securing more information about both the potential positives and negatives of flavors in youth initiation and getting adult smokers to quit or transition to potentially less harmful products. ‘Through this lens, the ANPRM we’re issuing today seeks comments, data, research results or other information on topics, including, but not limited to:- The role that flavors play in initiation and patterns of tobacco use, particularly among youth and young adults;
- The role that flavors may play in helping some adult cigarette smokers reduce cigarette use and/or switch to potentially less harmful tobacco products;
- The role that flavors in non-combusted tobacco products may play in quitting combusted tobacco products use, quitting all tobacco use or starting to use more than one type of tobacco product;
- Consumer perceptions of health risks and addictiveness of flavored tobacco products;
- Whether certain flavors used in tobacco products present potential adverse health effects to users or others; and
- The impact of local, state and international efforts to restrict the sale or marketing of flavored tobacco products.
Gottlieb’s piece is at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm601690.htm

Smokers detrained
Passengers in China who are discovered smoking on high-speed trains or in smoke-free zones of other trains will be prevented from train travel for 180 days, according to a story in The China Daily citing a newly-released document and a Guangzhou Daily report.
The document was issued jointly by the National Development and Reform Commission, the Supreme People’s Court and other government departments.
‘Troublemakers, producers and sellers of counterfeit tickets, and those endangering railway transport safety, will also be prevented from taking trains for 180 days,’ the China Daily story said.
‘A roster of those banned from taking trains will be publicized on 12306.cn, a ticket booking website, and creditchina.gov.cn, a credit check website, for seven consecutive days from the first day of each month. Those listed can appeal during that period.’
The rules are due to come into effect on May 1.
Last week, Reuters reported that China had said it would begin applying its ‘social credit’ system to trains and flights, under which people who had committed misdeeds would be barred from taking such transport for up to a year.
A story by Paul Huang for The Epoch Times, meanwhile, described the ‘social credit’ system as an experiment that international observers had speculated would be the next step in China’s transformation into a total surveillance state.