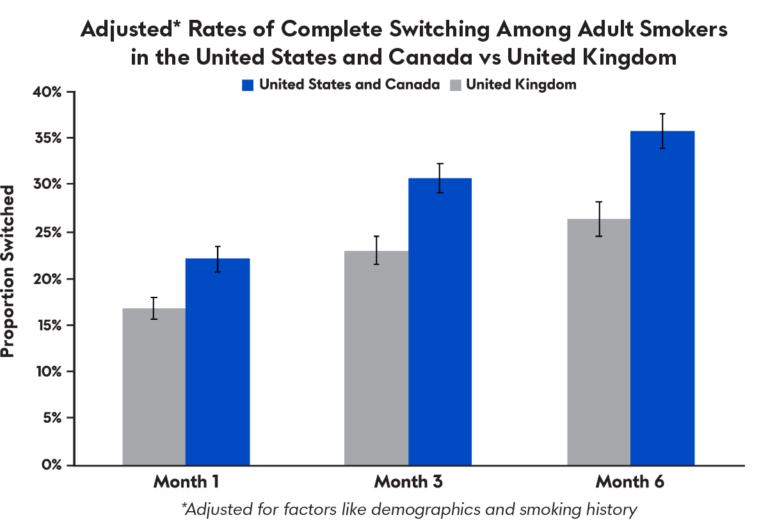
E-cigarettes that deliver a cigarette-like amount of nicotine are associated with reduced smoking and reduced exposure to a major cancer-causing chemical in tobacco, even with concurrent smoking, according to a new study led by researchers at Penn State College of Medicine and Virginia Commonwealth University (VCU).
“We found that e-cigarettes that delivered a similar amount of nicotine as traditional combustible cigarettes helped reduce smoking and exposure to a harmful carcinogen,” said Jonathan Foulds, a researcher at Penn State Cancer Institute and professor of public health sciences and psychiatry and behavioral health. “This study shows that when smokers interested in reduction are provided with an e-cigarette with cigarette-like nicotine delivery, they are more likely to achieve significant decreases in tobacco-related toxicants, such as lower exhaled carbon monoxide levels.”
The researchers conducted a randomized controlled trial of 520 participants who smoked more than nine cigarettes a day, were not currently using an e-cigarette device and were interested in reducing smoking but not quitting.

We found that e-cigarettes that delivered a similar amount of nicotine as traditional combustible cigarettes helped reduce smoking and exposure to a harmful carcinogen.
Jonathan Foulds, professor of public health sciences and psychiatry and behavioral health
According to Foulds, the findings represent an important addition to the scientific literature because they suggest that when e-cigarettes deliver nicotine effectively, smokers have greater success in reducing their smoking and tobacco-related toxicant exposure. Caroline Cobb, associate professor of psychology at VCU and lead author, said the study is important for two reasons.
“First, many e-cigarettes have poor nicotine-delivery profiles, and our results suggest that those products may be less effective in helping smokers change their behavior and associated toxicant exposure,” Cobb said.
“Second, previous randomized controlled trials examining if e-cigarettes help smokers change their smoking behavior and toxicant exposure have used e-cigarettes with low or unknown nicotine delivery profiles,” Cobb said. “Our study highlights the importance of characterizing the e-cigarette nicotine delivery profile before conducting a randomized controlled trial. This work also has other important strengths over previous studies, including the sample size, length of intervention, multiple toxicant exposure measures and control conditions.”
The study contributes to the ongoing question of what role e-cigarettes play in changing smoking behavior, and the findings support limited safety concerns for the use of the specific e-cigarette and liquid combinations over the short term, even in the context of concurrent cigarette smoking. However, Cobb added, very little is known about the effects of e-cigarettes over the course of years as opposed to the study’s 24-week period.