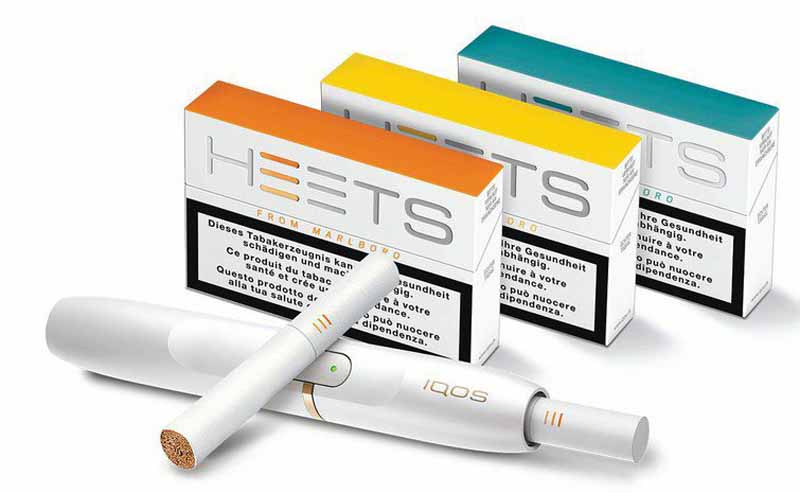
Philip Morris International announced today the ‘positive results from a new clinical study on IQOS, the company’s most advanced smoke-free product’.
‘The Exposure Response Study (ERS) measured the biological response of people who switch to IQOS for six months compared with continued smoking,’ the company said in a note posted on its website.
‘The study met its primary objective, demonstrating that after six months, eight measures of biological response (the primary clinical risk endpoints) improved in those who switched to IQOS.
‘PMI’s Scientific Assessment Program has rigorously tested IQOS over more than seven years and supports that switching to our most advanced smoke-free product is likely to present less risk of harm than continuing to use cigarettes. ‘Numerous aerosol chemistry and physics measurements demonstrate that IQOS aerosol contains an average of 90-95 percent lower levels of harmful constituents. Our results support that these reduced emissions translate to reduced toxicity in the laboratory and to reduced exposure in clinical studies.
‘The ERS contributes an important new facet to PMI’s research: it begins to explore the impact of these promising results by measuring the biological response of people who switch to IQOS compared with those who continue to smoke.’
“These results are very encouraging,” said Frank Luedicke, PMI’s chief medical officer. “We believe this study on IQOS is the first ever clinical study of this magnitude to directly assess the risk-reduction potential of a smoke-free product in people who switch to it.
“Everything we’ve seen, including these new results, continues to point in the direction of risk reduction.
“We are sharing the results with the scientific community at multiple conferences over the next few months and we look forward to their feedback.”
PMI said that on June 8 it had submitted the ERS results to the US Food and Drug Administration to add to the extensive body of evidence already presented to the agency in support of PMI’s pending application for authorization of IQOS as a modified risk tobacco product.
‘FDA is in the process of reviewing both PMI’s modified risk tobacco product and our premarket tobacco applications, but has not yet reached conclusion,’ it said.
Category: Science

More evidence on IQOS

Smoking revisited
In the last few decades, the lung cancer rate in China has risen sharply, but the culprit is not smoking; it’s pollution.
This is according to a piece by Robert Hoffman posted on the American Thinker website.
‘The war on tobacco, breathlessly waged by liberals and others who yearn to command everyone and everything, is based on the hysteria that smoking and being around the reprobates who smoke, as the surgeon general has declared, kill us,’ he said. ‘That countless other things do is dismissed as a distraction, not germane to the clear scientific facts.
‘A look at the clear scientific facts actually conveys quite a different conclusion.
‘According to nearly all the studies done, as opposed to the mere assertions, about 10 percent of lifelong cigarette-smokers contract any stripe of cancer. Those who consume three or more packs a day have a three-to-four-percent higher rate of lung cancer than the non-smoking population.
‘And among that non-smoking population, 10 to 15 percent are likely to get cancer of anything. ‘Thus, it would appear that in the absence of post hoc ergo propter hoc, you are more likely to be felled by cancer if you don’t smoke.’
Hoffman goes on to say that during the past few decades, the lung cancer rate in China has risen sharply, but that the culprit is not smoking.
The culprit is, he says, pollution, and the rate is considerably higher in the country’s smog-ridden cities.
BAT investing in glo
British American Tobacco said yesterday that it would invest €800 million during the next five years in its factory in Ploiești, Romania.
‘The investment, which will generate 200 new jobs in Romania, will support the expansion of BAT’s innovative tobacco heating product – glo – in countries across Europe during the second half of 2018,’ the company said in a press note posted on its website.
‘A completely new manufacturing hall will be built dedicated to producing the specially designed tobacco sticks – called Neostiks – which work with the glo tobacco heating device. In total, an additional 7,000 square metres of production space will be created and, once completed, the Romanian production plant will be the sole supplier of glo Neostiks across Europe, as BAT continues its ambition to transform the tobacco industry with a range of potentially reduced-risk alternatives for smokers.’
The investment will enhance also the cigarette-making capabilities within the factory.
“We have a long-held ambition to offer smokers a range of potentially reduced-risk products – like tobacco heating devices, e-cigarettes and oral tobacco,” Tadeu Marroco, regional director – Europe and North Africa, was quoted as saying. “This ambition has seen us launch vaping products and tobacco heating devices in 16 countries in the last five years and we’ve bold plans to increase our geographical footprint in the second half of 2018. The significant investment in our factory in Romania is testament to our commitment to offer smokers a wider range of tobacco and nicotine products – with a particular focus on potentially reduced-risk alternatives to smoking – in an increasing amount of countries.”
The factory will supply Neostiks also for the Romanian market following the launch of glo there in December. In the six months since its launch, first in Bucharest and then in 17 other major cities around Romania, almost 25,000 consumers are said to have bought glo and tens of millions of Neostiks have been sold.
In 2017 BAT said, its revenues outside of the US from e-cigarettes and tobacco heating products quadrupled to £397 million. On a full year basis including Reynolds American’s contribution, this would have been more than £500 million.
And the company aims to generate more than £1 billion revenue from NGPs by the end of 2018 and to more than £5 billion by 2022.
Saving lives
A leading US public health expert has criticized two researchers at the Johns Hopkins Bloomberg School of Public Health for denying that smoking is known to be more hazardous than is vaping.
According to Dr. Michael Siegel (pictured), a Professor in the Department of Community Health Sciences, Boston University School of Public Health, the researchers made the denial in an article published in the Summer 2018 issue of the Hopkins Bloomberg Public Health Magazine. Dr. Ana Maria Rule, an assistant professor in the Department of Environmental Health and Engineering, was said to have argued that: ‘Even if vaping proves safer than smoking, that’s still a long way from a gold stamp for their safety’.
And Dr. Joanna Cohen, a professor and director of the Institute for Global Tobacco Control, was quoted as stating: ‘They are likely safer than continuing to smoke combustible cigarettes, but without the long-term studies, we just don’t know’.
Writing on his blog, The Rest of the Story, Siegel said that the problem with this denialism was not merely that it spread misinformation. ‘The problem is that this is exactly the kind of false propaganda that is deterring many smokers from trying to quit smoking using vaping products and is causing some ex-smokers to return to smoking,’ he said.
‘Whether they realize it or not, this is precisely the effect statements like those being made by these Johns Hopkins researchers are having on the public. In fact, several national surveys have demonstrated that the public is largely misinformed about the relative hazards of smoking vs. vaping. And it is this misperception that has stunted what otherwise could have been a much more substantial shift from smoking to vaping in this country. In other words, this isn’t just a question of misleading the public. It’s a question of saving lives, or failing to do so.
‘Hopefully, these researchers will publish a correction or retraction of these claims so that we can begin the process of restoring some semblance of a science base in the field of tobacco control.’
'Clarification' sought
A statement made last week by the Korean Food and Drug Administration (KFDA) could mislead millions of people into thinking that the use of heated tobacco products is as harmful as smoking cigarettes, according to Philip Morris International.
‘There is compelling scientific evidence, including KFDA’s own findings, that heated tobacco products generate an aerosol that is completely different from cigarette smoke, and that they are a better choice than cigarettes, a PMI press note said.
‘And yet, with their June 7 statement, the agency could be risking the health of millions of Koreans who use tobacco products. These are the very people they are charged with protecting.
‘Those who are still smoking may be discouraged from switching, and those who have switched may turn back to cigarette smoking.’
PMI said that everybody agreed that smoking was harmful. Now, it said, there was a much better alternative, made possible by technology and science, for the millions of Koreans who would put themselves at the greatest risk of serious health consequences if they continued to smoke cigarettes.
‘Rather than focusing on the significant reductions in harmful chemicals compared to cigarette smoke that the agency’s own science shows, KFDA points to “tar” measurements to judge the relative risk of tobacco products,’ the note said.
‘However, the World Health Organization (WHO) – an objective and respected group with the public good as its goal – has been crystal clear on this matter: “Tar need not be measured, as it is not a sound basis for regulation, and the levels can be misleading”. Exactly. The concept of “Tar” applies to cigarette smoke, which is not the same as the aerosol from heated tobacco products – a fact that has been confirmed by numerous studies.
‘Scientific evidence shows that switching to heated tobacco products, while not risk-free, is a much better choice than continuing to smoke. ‘Koreans who use tobacco products, and those around them, deserve truthful information based on 21st-century science – not political agendas. Measuring “tar” is yesterday’s approach misapplied to today’s innovative products.
‘For the benefit of the people of Korea, we believe KFDA should consider issuing a clarification.’
High nicotine levels needed
Vapers using low- rather than high-nicotine e-liquids in electronic cigarettes may be using their devices more intensely, potentially increasing the risk of exposure to toxins in the vapor, according to a medicalxpress.com story citing new research funded by Cancer Research UK and published in Addiction on June 7.
Researchers, based at London South Bank University, studied 20 e-cigarette users and found that people using low-nicotine e-liquid in their devices puffed more deeply and more often than did those using high-nicotine liquid. Those using low-nicotine liquids also increased the power of their vaping devices when possible.
Despite this ‘compensatory’ behaviour, the low nicotine vapers were unable to get as much nicotine as could the high-nicotine group. But in their quest to do so their puffing behavior may have increased their exposure to toxins such as formaldehyde, a chemical formed when the e-cigarette liquid is heated.
While there can be toxic chemicals present in vapor, they are far fewer and generally at lower concentrations than in tobacco smoke. Evidence so far still shows that the use of both high- and low-nicotine e-cigarettes is far less harmful than is smoking.
“Some vapers might believe that starting out on a low nicotine strength is a good thing, but they should be aware that reducing their nicotine concentration is likely to result in the use of more e-liquid,” said Dr. Lynne Dawkins, lead author of the study.
“This obviously comes with a financial cost but also possibly with a health cost. The results of our study suggest that smokers who want to switch to vaping may be better to start with higher, rather than lower, nicotine levels to reduce compensatory behaviour and the amount of e-liquid used.”
PMI issues research update
Philip Morris International yesterday published its latest Scientific Update for Smoke-Free Products, a regular publication on its research efforts to develop and assess a range of smoke-free alternatives to cigarettes.
‘We are focusing this issue on the growing body of independent research on our electrically heated tobacco product (EHTP, marketed as IQOS), the most advanced smoke-free alternative in our portfolio,’ according to a press note posted on the company’s website.
‘The covered research comprises independent, peer-reviewed publications on smoke-free products that focus on EHTP, and also includes four recent government reports from the UK, the US, Germany, and the Netherlands.’
Other sections are said to include an update on the assessment of each product in PMI’s smoke-free portfolio, recent R&D milestones and a compendium of the company’s peer-reviewed publications on its smoke-free products this year.
“We are happy to see growing interest in studying our smoke-free products and that the general trend among independent results is in line with our own research,” said Prof. Manuel Peitsch, PMI’s chief scientific officer.
“Independent research on our electronically heated tobacco product, ETHP, demonstrates significant improvements relative to cigarettes, and is crucial to our efforts to change the lives of millions of smokers around the world.”
PMI said its extensive research and assessment program was inspired by the well-recognized practices of the pharmaceutical industry and in line with the draft guidance of the US Food and Drug Administration for Modified Risk Tobacco Product (MRTP) Applications.
HNB probe results due
South Korea’s health authorities have indicated that they will announce this month the findings of their investigation into whether heat-not-burn (HNB) products deliver potentially harmful substances.
The Ministry of Food and Drug Safety said it would make its announcement before June 13.
The ministry launched its investigation in August into three HNB devices – Philip Morris Korea’s IQOS, British American Tobacco’s Glo, and KT&G’s lil.
‘The investigation is focused on the amount of harmful chemicals such as nicotine and tar released in e-cigarettes,’ the story said.
World No Smoking Days
Philip Morris International is marking World No Tobacco Day, May 31, with a call to recognize it as ‘World No Smoking Day’.
PMI said in a note posted on its website that it was publishing informational advertisements highlighting that people who smoke deserve the opportunity to learn about smoke-free alternatives to cigarettes.
‘It is clear that despite the well-known health risks associated with smoking, many people continue to smoke,’ it said. ‘The World Health Organization (WHO) predicts that there will be more than one billion people who smoke in 2025, about the same number as today.
‘Their best choice would be to quit – but many don’t. It makes sense that these men and women should have access to and information about less-harmful alternatives to cigarettes.
‘Why would anyone deny them this opportunity?’
PMI said it was calling for an additional, bold approach to public health.
“For people who will otherwise continue to smoke, WHO is in the perfect position to drive switching from cigarettes to sensible alternatives,” said André Calantzopoulos, PMI’s CEO.
“A policy that informs people about those alternatives – in essence, a World No Smoking Day – would reduce smoking prevalence to a far greater extent and at a much faster rate than the existing suite of tobacco-control measures alone.
“People who smoke deserve a sensible plan that takes full account of better alternatives to cigarettes.
“For our part, we’re determined to deliver a smoke-free future through innovations that stand up to scientific scrutiny and that meet consumer needs.”
PMI said it had already committed $4.5 billion in supporting a team of 400 world-class scientists, engineers and technicians who had spent years creating and testing a range of smoke-free products that offered a much better choice for the millions of smokers who didn’t quit.
It said that more than five million smokers worldwide had already completely abandoned cigarette smoking and switched to IQOS, PMI’s heated tobacco product, with 10,000 smokers switching every day. And PMI added that it was not the only company pursuing innovation as important to the world’s one billion smokers worldwide – the tobacco industry generally is beginning to move in this direction, to the benefit of men and women who smoke.
“Our short-term ambition is that one out of three of our consumers, 40 million men and women who smoke, will have switched to better alternatives by 2025,” said Calantzopoulos. “Ultimately, we want to be in a position to stop selling cigarettes entirely. However, we need the support of governments and the public health community to make this happen in as short a time as possible. I believe that instead of just designating one day as World No Tobacco Day, we should promote every day as World No Smoking Day.”
PMI said it was proposing that governments and authorities investigate thoroughly how scientifically substantiated smoke-free products could be used as a complementary public health strategy alongside smoking prevention and cessation. With appropriate government control and oversight, these products could have a meaningful and positive impact on public health, it added.
More information about PMI’s views on World No Tobacco Day is at: http://www.pmi.com/world-no-smoking-day-2018.
Heated tobacco warning
A scientist and health advocate says it is important that the European Parliament is presented with the available evidence that heated tobacco products ‘remain both harmful and highly addictive’.
Writing an opinion piece in The Parliament Magazine, Professor Charlotta Pisinger said that no product that damaged the lungs and human health should be promoted to young people across the EU, especially in the ways these new products were presented.
‘As Chair of the European Respiratory Society (ERS) Standing Committee on Tobacco Control, I will present my research findings to the European Parliament in Strasbourg on the 30th May, alongside MEP Gilles Pargneaux and the European Network for Smoking and Tobacco Prevention (ENSP), on the occasion of World No Tobacco Day,’ she said.
‘Independent research suggests that there is a substantially higher health risk than claimed by the tobacco industry.
‘I have discovered that tobacco companies have not informed the public that some harmful substances – such as particulate matter, tar, acetaldehyde (a carcinogen), acrylamide (a potential carcinogen) and an acrolein metabolite (toxic and irritant) – were found in high concentrations in their studies.
‘Some studies also found much higher concentrations of formaldehyde (a potential carcinogen) in heated tobacco products than in conventional cigarettes.’
New Heated Tobacco Products: No Smoke, No fire? hosted by MEP Gilles Pargneaux is due to be held from 13:30 to 15:30 on May 30 at the European Parliament in Strasbourg, room Louise Weiss N3.5. Registration is at: https://forms.ersnet.org/new-heated-tobacco-products.