A study has found that the levels of three compounds in e-liquid emissions were hugely lower than those reported in a previous study, according to a ScienceDirect.com story relayed by the TMA.
The replication study, conducted by a team of researchers led by Dr. Konstantinos E. Farsalinos at the Onassis Cardiac Surgery Center and the University of Patras in Greece, is scheduled to be published in the May edition of the journal Food and Chemical Toxicology.
It tested three e-liquids from a previous study (in standard and sweetened versions) using the same device and puffing patterns, and found a 589-fold decrease in the levels of formaldehyde (8.3–62 μg/g), acetaldehyde (12.1–26.0 μg/g) and acrolein (5.4–19.4 μg/g) from those reported as part of the earlier study.
The earlier study had identified aldehyde emissions up to 10,000-times higher in flavored e-liquids than in unflavored e-liquids.
But the latest study found that aldehyde emissions from all flavored e-liquids were 79.0–99.8 percent lower than those associated with smoking.
And they were lower than commonly measured indoor levels and occupational and indoor safety limits.
The researchers said the devices tested emitted very low levels of aldehydes, and that while some flavorings might contribute to aldehyde emissions, the absolute levels were minimal.
Category: Science

Emissions reassessed

Research has real bite
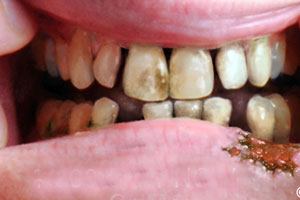
A study undertaken by British American Tobacco has shown that teeth exposed to cigarette smoke over a period of two weeks became ‘very discolored very quickly’, whereas teeth exposed to e-cigarette vapor or vapor from glo, a heat-not-burn product, did not. After two weeks of almost continuous exposure, the teeth exposed to vapor were almost indistinguishable from teeth that were not exposed to anything at all.
‘Smokers get “stains”, turning teeth from a healthy-looking white to an unhealthy-looking yellow/brown color,’ BAT said in a press note. ‘Although this staining is commonly referred to as nicotine staining, it is not caused by nicotine, but by tar in smoke.
‘As part of a wider study on oral health, scientists at British American Tobacco studied discoloration in teeth. A prototype Vype e-cigarette and a tobacco heating product, glo, were assessed for tooth discoloration and the impact on teeth compared to that of cigarette smoke.
‘A puffing robot was used to puff on the products to produce smoke and vapor. In each case, the smoke or vapor was collected onto a filter pad (Figure 2B) and then a solvent was used to extract the solid material from the filter pad. The impact of the extracted material (extract) on tooth discoloration was then tested using cows’ teeth.
‘Cows’ teeth are routinely used in laboratory experiments in lieu of human teeth. They are used for example to test oral hygiene products like toothpaste and mouthwash.
‘The teeth were prepared by polishing them using fine sandpaper to create a surface more like that of human teeth. The teeth were then incubated at body temperature in human saliva to create an environment mimicking that in the human mouth. This incubation results in the creation of the so-called pellicle layer on the teeth, which is the smooth film you can feel on your teeth when you rub your tongue over them. It is the normal protein layer that forms on teeth when certain molecules in saliva bond to the tooth enamel.
‘The teeth were incubated in an oven at body temperature and exposed to the various extracts from the cigarette smoke or e-cig or THP vapor. Some teeth were also incubated in solvent with no extract at all to act as a control/blank.
‘After the first day, the teeth exposed to the smoke extract started to change colour and over the course of 14 days, these teeth got darker and darker in color. Even with the naked eye, the color changes with the cigarette extract could be easily seen after one day. The exact color change was also measured in the laboratory using a special camera that can detect tiny changes in hue. In contrast to teeth exposed to smoke those exposed to e-cigarette or THP vapor exhibited minimal change in color, similar to untreated teeth.’
These results were presented on Saturday at the annual conference of the American Association for Dental Research in Florida, US.
More evidence on vapor
New data presented at the Society of Toxicology Annual Meeting (SOT) have demonstrated the potential risk reduction offered by switching from conventional cigarettes to two different heat-not-burn tobacco products and e-cigarettes.
A PMI Science press note issued on Wednesday said that a range of studies had investigated the toxicological impact of Philip Morris International’s Tobacco Heating System 2.2 (THS 2.2), Carbon Heated Tobacco Product 1.2 (CHTP 1.2) and prototype e-cigarette products in the context of respiratory disease, cardiovascular disease, and lung cancer using several different approaches. In each case, the aerosols produced by the alternative products were said to have resulted in significantly reduced levels of biological impact as compared to cigarette smoke (CS).
‘One six-month, multi-arm exposure study compared the effects of CS with those of the aerosol from the two heat-not-burn tobacco products THS 2.2 and CHTP 1.2 using a mouse model,’ the note said. ‘In both THS 2.2 and CHTP 1.2, tobacco is heated rather than burned, resulting in significantly reduced levels of harmful chemicals emitted and inhaled as compared with CS,’ the note said.
‘Through a systems toxicology approach, combining physiological, histological, and omics [various biology disciplines] endpoints, the study found that exposure to THS 2.2 and CHTP 1.2 aerosols had minimal adverse respiratory and cardiovascular effects in comparison to exposure to CS. The findings are in line with a previous assessment of THS 2.2, demonstrating reproducibility of the results obtained. In addition, both cessation and switching to CHTP 1.2 aerosol exposure after three months exposure to CS reversed inflammatory lung responses, halted the progression of aortic plaque growth and reduced the perturbations of biological pathways in heart tissue, to levels typically seen following exposure to fresh air alone.’
PMI Science said that another study presented at SOT had assessed lung inflammation, emphysema and the underlying molecular changes typically associated with lung cancer following up to 18 months of exposure to either CS or THS 2.2 aerosol. ‘The study used a combination of traditional toxicology endpoints as well as systems toxicology techniques including histological, transcriptomic, and proteomic analysis of the lungs,’ the note said. ‘In all endpoints, the biological impact of THS 2.2 aerosol was significantly lower than that of CS.’
Meanwhile, the company said that, in line with the principles of 21st century toxicology, PMI was developing also novel in vitro methods for toxicity testing using human cells. ‘Such models have the potential to reduce the necessity for animal testing and offer more cost-efficient and timely results, as well as a detailed understanding of the biological processes underlying toxicity,’ it said.
‘In collaboration with the Institute for In Vitro Sciences (IIVS), one study presented at the SOT evaluated the performance and reproducibility of three new in vitro assays. Six laboratories conducted comparison of the assays and found that these non-animal test systems may provide consistent human-relevant data corresponding to key events involved in respiratory disease. A further in vitro methodology using human bronchial epithelial cells was used in a study assessing the effects of THS 2.2 aerosol and CS.’
“The multi-lab comparison of these non-animal systems paves the way for more robust and meaningful strategies for toxicity testing,” said Dr. Holger Behrsing, principal scientist, IIVS. “They allow us to generate human-relevant data that will be of interest not just to industry and research scientists, but also to regulatory bodies. In order to develop these assays and ensure they reach their fullest potential, collaboration is key. Working with PMI and a range of different laboratories has allowed us to leverage expertise across the field and demonstrate the reproducibility of our findings. In the spirit of open science, we hope that this will open the door to further collaborations in the investigation, development, and validation of novel in vitro systems.”
In the assessment of three prototype e-cigarettes, biological changes following three-week’s exposure to the either the e-cigarette aerosols or CS were assessed using traditional and systems toxicology endpoints. CS exposure was found to induce biological responses associated with smoking-related diseases in the respiratory tract, while e-cigarette aerosol exposures, even at higher levels of nicotine delivery, resulted in substantially reduced molecular and microscopic changes. Two additional e-cigarette studies assessed the biological effects of flavor compounds typically added to e-cigarette liquids, demonstrating the potential to establish a scientifically substantiated list of minimally-toxic flavor ingredients.
PMI said it was leading a full-scale effort to ensure that heat-not-burn tobacco products and e-cigarettes ultimately replaced cigarettes.
Further information on PMI’s Reduced-Risk Product development and assessment program can be found at www.pmiscience.com.
Turning the HEETS up
Philip Morris International said yesterday that it had stopped cigarette production at the Aspropyrgos factory of its Greek affiliate, Papastratos, which was now exclusively producing HEETS, the tobacco units used with its heated-tobacco product, IQOS.
‘This first full conversion of a cigarette factory is a landmark step in our vision of a smoke-free future where people who smoke switch from the most harmful form of nicotine consumption – cigarettes – to scientifically substantiated smoke-free alternatives,’ the company said in a note posted on its website.
‘The €300 million investment included the construction of three new buildings and the replacement of cigarette production lines with high-tech facilities capable of producing 10,000 smoke-free tobacco units per minute.
‘The conversion of the factory started in August 2017. The facility is expected to be fully operational by the end of 2018 and will create 400 new jobs.’
“This is a historic day for our company,” said André Calantzopoulos, PMI’s CEO. “Papastratos is the first of our factories to end cigarette production and fully shift to manufacturing our smoke-free alternatives.
“We will continue to convert existing sites and invest in new facilities to answer global adult smoker demand for better alternatives to cigarettes.
“We made a commitment to provide all people who would otherwise continue smoking with potentially less harmful products. The momentum around this revolutionary change for the benefit of the world’s 1.1 billion smokers, public health and society at large is growing, and we will continue working towards a smoke-free future.”
Along with PMI’s plant near Bologna, Italy, Papastratos is its second facility fully dedicated to manufacturing smoke-free products. PMI has announced plans also to transform, either fully or partially, its cigarette factories in Korea, Romania and Russia.
‘Since 2008, we have invested more than US$4.5 billion in scientific research, product and commercial development, and production capacity related to IQOS and other smoke-free products,’ the note said. ‘In 2017, over 70 percent of our global R&D expenditure and over 30 percent of our global commercial expenditure was allocated to smoke-free products.
‘We estimate that at the end of January 2018, nearly five million adult consumers around the world have already stopped smoking and switched to IQOS. Our ambition is that all those who would otherwise continue smoking abandon cigarettes and switch completely to scientifically substantiated smoke-free products as soon as possible. Appropriate regulatory policies and decisions can substantially accelerate the speed and magnitude of this historic change.’
Evidence building
A clinical study conducted by scientists at British American Tobacco has revealed that when smokers switch completely from cigarettes to the heated-tobacco product, glo, their exposure to certain cigarette smoke toxicants is significantly reduced, in some cases to levels comparable to those seen in smokers who quit smoking completely.
In a press note issued today, BAT said these results added to evidence suggesting that glo may ‘have the potential to be substantially reduced risk compared to smoking conventional cigarettes’.
‘Because glo vapor has lower levels of toxicants than cigarette smoke, it should in principle expose consumers to much less toxicants,’ the note said. ‘The results of this study indicate that this is indeed the case.’
The clinical study was conducted in Belfast, UK, over seven days and involved 150 people, all of whom were smokers for at least three years prior to enrolment.
‘For the first two days, study participants continued to smoke as normal and their urine was collected to measure levels of chemicals. Blood and breath were also collected for analysis,’ the note said.
‘For the next five days, participants were randomly allocated to either continue smoking, switch to using a THP [tobacco heating product] or quit smoking. Urine, blood and breath samples were again collected for analysis.
‘Exposure to certain smoke toxicants was determined by measuring the levels of certain chemicals in the urine. These could be the toxicants themselves or their metabolites – which is what the body breaks it down into – called biomarkers of exposure. Toxicants measured included those identified by the World Health Organization as being of concern in cigarette smoke.’
The results were said to have shown that the concentration of certain chemicals in the urine was reduced in smokers who switched to glo. In some cases, these reductions were the same as those observed in the smokers who quit.
“These results are very encouraging,’ said Dr. James Murphy, head of reduced risk substantiation at BAT. “The next step will be to determine whether this reduction in exposure translates to a reduced biological effect, and in turn a reduction in adverse health effects for those smokers who switch completely to glo.”
BAT said that future clinical studies would test for markers of biological effect, such as cholesterol levels or heart rate – measurements that give an indication of general health. A reduction in biomarkers of biological effect could suggest that a reduction in exposure is having a positive impact on reducing the adverse health risks of smokers who switch completely.
“The results of one test are important,” said Murphy, “but it is the combination of the results of many different tests that start to give us a real feel for the bigger picture and the potential for glo to be reduced risk compared to a conventional cigarette.”
The results of the clinical study are being presented today at the annual conference of the Society of Toxicology in San Antonio, Texas, US.
Reducing risk
A new laboratory-based study has shown that when airway cells damaged by cigarette smoke are exposed instead to vapor from the heated-tobacco product, glo, some of the biological effects caused by the smoke exposure are reversed.
Basically, subsequent exposure of cigarette-smoke-impacted cells to glo vapor had a similar effect to their subsequent exposure to air – the cells were able to maintain their ability to repair themselves.
In a press note, British American Tobacco said that the vapor produced by glo contained about 90-95 percent less of certain toxicants than did cigarette smoke.
The company said that previous studies had shown that the biological impact of glo vapor on cells tested in the laboratory was much less than the impact of cigarette smoke on similar cells. But, it added, the reversibility of the damage following switching to a product such as glo had not been extensively studied.
“Products like glo are very new, so understanding the biological impact of vapor from glo and how that compares to cigarette smoke is a core component of our scientific research,” said Dr. James Murphy, head of reduced risk substantiation at BAT.
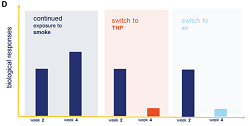
In the study, human airway cells were exposed repeatedly to either cigarette smoke or vapor over four weeks. For the first two weeks, the lung tissue was exposed to cigarette smoke for 15 minutes at a time, three times a week. The exposed tissue was then split into three groups: one group continued to be exposed repeatedly only to cigarette smoke for a further two weeks; a second group was exposed repeatedly to glo vapor and the third group was exposed only to air. The results obtained were then compared to results obtained by exposing airway tissue only to air for the full four weeks.
‘The results show that switching completely to glo after two weeks of repeated exposure to cigarette smoke reversed some of the biological impacts of the smoke,’ the press note said. ‘Significant reductions were observed in the amount of certain molecules produced in response to inflammation, for example, in comparison to that seen in lung tissue exposed to cigarette smoke for the full four-week period.’
BAT said the results added to evidence suggesting that glo might have the potential to be reduced risk compared to conventional cigarettes.
“We have developed a suite of tests to assess our next generation products, because we know it is by taking the results of all these tests together that gives us a real feel for the bigger picture and the potential for glo to be reduced risk compared to a conventional cigarette,” said Murphy.
The results are being presented today at the annual conference of the Society of Toxicology in San Antonio, Texas, US.
CORESTA calls for papers
The organizers of the 2018 CORESTA Congress yesterday called for papers.
The Congress, whose theme is Science and Innovation: Addressing the needs, will be hosted by the China National Tobacco Corporation (CNTC).
It is due to be held on 22-26 October at the Intercontinental Hotel, Kunming, China.
In a joint announcement, the CNTC Congress Organizing Committee and the CORESTA Secretariat said that the call for papers was now online.
The organizers said the call for papers was currently accessible through the CORESTA website at www.coresta.org and would be made available later through the official Congress website.
Direct access to the abstract submission system was available through the CORESTA website at: CORESTA 2018 abstract submission
The announcement said authors would receive immediate receipt messages by email to confirm the successful submission of their abstracts and would be informed of the CORESTA Reading Committee’s selection towards the end of June 2018.
The deadline for the submission of abstracts is May 16.
Another glowing report
A clinical study conducted by scientists at British American Tobacco has revealed that when smokers switch completely from cigarettes to the tobacco-heating product (THP) glo, their exposure to certain cigarette smoke toxicants is significantly reduced, in some cases to levels comparable to those seen in smokers who quit smoking completely.
The company points out in a footnote that the results of the study do not necessarily mean that glo is less harmful than are other tobacco products.
It says, however, that the results add to ‘evidence suggesting that [using] glo may have the potential to be substantially reduced risk compared to smoking conventional cigarettes’.
A BAT press note said that glo was designed to heat rather than burn tobacco, which meant that it did not produce smoke, and that certain toxicants associated with tobacco combustion were substantially reduced. Previous studies had revealed that toxicant levels in the vapor from glo were about 90-95 percent lower than they were in cigarette smoke.
“Products like glo are very new and consumers and regulators alike understandably want as much information as possible about them,” said Dr. James Murphy, head of reduced risk substantiation at BAT. “Understanding how vapor from glo compares to cigarette smoke is, therefore, a core component of our scientific research. Clinical studies, which are studies involving real people, are an extremely important component of that.”
The press note said that because glo vapor had lower levels of toxicants than did cigarette smoke, it should in principle expose consumers to much lower levels of toxicants.
The results of this study, which were due to be presented on Saturday at the annual conference of the Society for Nicotine and Tobacco Research in Baltimore, Maryland, US, indicated that this was the case.
Putting the record straight
Public Health England (PHE) is trying to dispel some of the persistent inaccuracies and misconceptions that surround electronic cigarettes and vaping.
Writing on the Gov.uk Blogs website, Martin Dockrell, PHE’s tobacco control programme lead, said that e-cigarettes tended to court controversy among the public and media alike. Not surprisingly, there were lots of inaccuracies and misconceptions about e-cigarettes and vaping.
‘Our latest comprehensive independent e-cigarette review, authored by leading academics in the tobacco control field, looks at the up-to-date international data and peer-reviewed research,’ Dockrell said.
‘Despite the sometimes confused, and confusing, media reporting around the safety of e-cigarettes, there is growing consensus around the evidence. While not without some risk, when compared to smoking e-cigarettes are far less harmful.
‘This view is supported by a number of key bodies, including Cancer Research UK, Action on Smoking and Health, the Royal College of Physicians, the British Medical Association and, recently, a major US science body, the National Academies of Sciences, Engineering, and Medicine.’
Dockrell then goes on to examine and debunk five common myths about e-cigarettes and vaping.
In summary, he said, e-cigarettes and tobacco cigarettes were not the same and shouldn’t be treated as such. ‘It’s important that England’s seven million smokers are aware of the differences and have accurate information to inform their health decisions. E-cigarettes aren’t completely risk free but carry a fraction of the risk of smoking and are helping thousands of smokers to quit and stay smoke-free.’
Dockrell’s blog is at: https://publichealthmatters.blog.gov.uk/2018/02/20/clearing-up-some-myths-around-e-cigarettes/
Canada in dialogue
The Global Forum on Nicotine (GFN) is due to stage a free-to-attend, tobacco-harm-reduction dialogue in Vancouver, Canada, in April.
The dialogue, Tobacco harm reduction: different strokes for different folks, or a consistent approach?, is to be held in partnership with the BC Centre for Disease Control and the Canadian Drug Policy Coalition.
It will be held from 09.00 to 16.30 on April 9, at the Morris J Wosk Centre for Dialogue in Vancouver.
‘The huge growth in the availability of safer nicotine products, with new technologies, such as vaping and heat not burn, as well as oral tobacco products, such as snus, has created greater opportunities for smokers to switch from a proven dangerous and unhealthy way to consume nicotine to much safer methods, according to a press note from the GFN, which has previously run series of dialogues in the UK and Ireland.
‘The emerging science surrounding both the technology and the products is positive and encouraging.
‘Vancouver has a proud tradition for supporting harm reduction for illicit drug use, including pioneering supervised consumption rooms for injecting drug use. The principles of harm reduction are well understood and have been enacted for many years with positive results.’
Participants will be addressed by international and local presenters, including:- Dr. Mark Tyndall, executive medical director, BC Centre for Disease Control;
- Professor Marjorie MacDonald, School of Nursing, University of Victoria;
- Professor Gerry Stimson, professor emeritus, Imperial College, London;
- Jacques Le Houezec, independent consultant in public health and tobacco dependence, France;
The presenters are due to examine:
- The history of harm reduction in Vancouver, the lessons learnt and the implications for this approach in relation to tobacco and smoking.
- Tobacco harm reduction as the ‘new kid on the block’ and what the emerging evidence is telling us.
- What does regulation look like and what are the elements that make for appropriate and effective regulation?
- The consumer experience – what products do people use and what are the results for them?
- What are the key issues for policy-makers and how can we ensure buy-in from all stakeholders?
Attendance at the dialogues is free, but participants are required to register at: https://gfn.net.co/dialogues/register.
More details about the dialogue are at: https://gfn.net.co/dialogues/vancouver-2018.