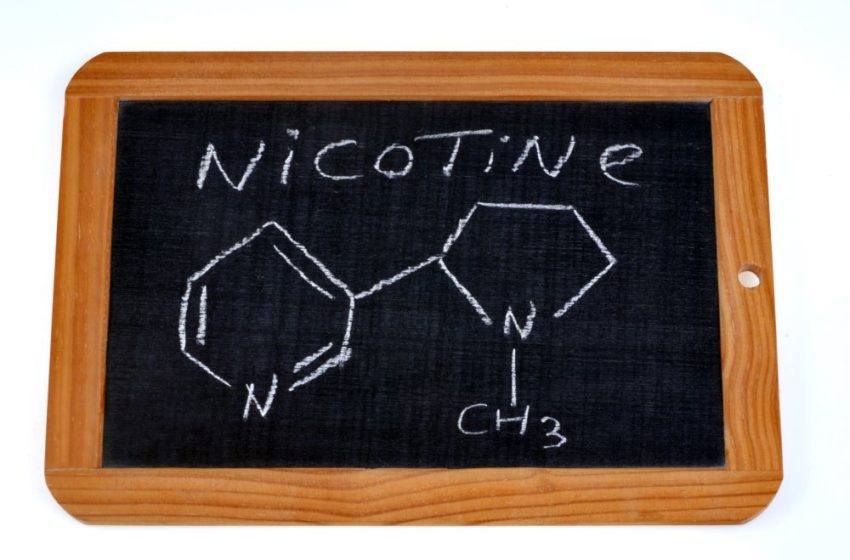
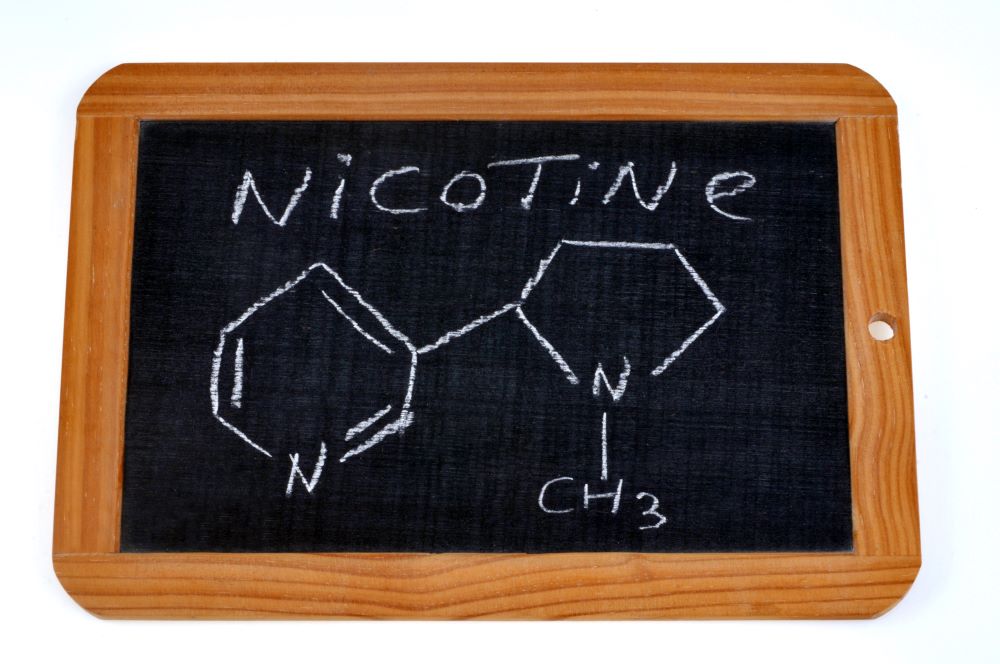
A new scientific study on Vuse underscores the contribution vapor products can make toward tobacco harm reduction.
By James Murphy
Almost 20 years ago, I joined BAT as a scientist motivated by the positive change that tobacco harm reduction (THR) can achieve. We have made great strides since then, and on days like World Vape Day, it is encouraging to see continued innovation in this area. This ambition to support THR is embodied by BAT’s release today of one of the largest vapor product studies ever conducted, further supporting the role that Vuse, BAT’s flagship vapor brand, can play in tobacco harm reduction.
Science and research have been fundamental to the progress that I have witnessed over almost two decades at BAT. Together, they form the cornerstone of consumer and regulatory confidence in our brands. This confidence is essential for our new-category products to be able to both support THR and provide reduced-risk*† alternatives for consumers who would otherwise smoke.
Early in my career, I had the opportunity to lead product development for BAT’s first vapor product, which would subsequently turn into Vuse: a billion-pound brand and the No. 1 global vaping brand by market share.1 Now, in 2023, there is wide acceptance that vaping continues to grow in importance as adult smokers seek reduced-risk alternatives to continued smoking.
As director of research and science at BAT, my priority is to develop and publish science-based information needed to better understand the real-world impacts of our products.
Our latest study, published today in the peer-reviewed journal Internal and Emergency Medicine, is intended to do just that. By comparing clinical measurements from exclusive Vuse consumers with current smokers, the results of the study show that adult consumers using BAT’s vapor brand Vuse2 had significantly better results for biomarkers relevant to smoking-related diseases than smokers.
For priority cigarette smoke toxicants identified by the World Health Organization, levels of exposure were significantly lower in the Vuse consumers compared to the smokers. Additionally, favorable differences between the Vuse consumers and smokers were found across all seven measured biomarkers of potential harm relevant to smoking-related diseases.
We believe these results underscore the contribution that vapor products can make toward tobacco harm reduction and how, by using a robust evidence base to substantiate the role of reduced-risk* products, we can give adult smokers who would otherwise continue to smoke access to satisfactory reduced-risk* alternatives.
While World Vape Day brings together a global community to converse and learn about tobacco harm reduction, there are significant barriers and challenges facing companies involved in the vaping industry. I believe a core component to overcoming these barriers is evidence-led and science-backed regulation and leadership. These two areas are key to ensuring that reduced-risk* products are only made available to adult consumers and that consumers feel confident about the quality and safety standards governing the development of alternatives like vapor products.
In countries where the concept of tobacco harm reduction has been embraced, we have seen accelerated declines in smoking rates as smokers migrate to noncombustible products. Sweden, for instance, is on the cusp of becoming the first European country to become officially smoke-free—with smoking rates at just 5.6 percent—a 51 percent drop in a decade. Low smoking rates have also had direct benefits for Swedish public health, with cancer rates that are 41 percent lower than the rest of Europe. However, many countries are yet to offer consumers legal access to reduced-risk* products as alternatives to widely available conventional cigarettes.
I am more convinced than ever that tobacco harm reduction has the potential to be transformative for our consumers and significantly lower currently projected smoking rates worldwide.
There is a clear opportunity to use the scientific evidence we now have available to substantiate the benefits of reduced-risk* products for those who would otherwise smoke. Looking ahead, I am increasingly confident that the quality of ongoing research from us, other manufacturers, academics and public health authorities—combined with open and honest debate with a diverse range of political, regulatory and public health stakeholders—will accelerate tobacco harm reduction.