The South Korean city of Guri has developed a machine that transforms cigarette butts into compost, according to a story in The Korea Bizwire.
The technology is being tested in the grounds of government offices, where the machine is housed inside a smoking booth.
According to the story, the process uses microorganisms harmless to humans to create usable compost out of the butts – compost that has the added advantage of repelling pests.
The authorities in Guri have indicated they will place cigarette-butt composting-machines around the city after they have concluded the initial trial and conducted an overview of the results.
Category: Technology

Butts into compost

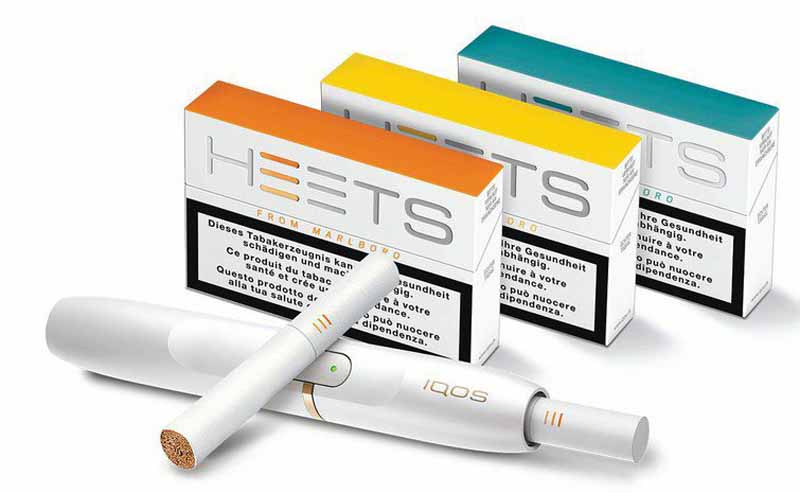
Turning the HEETS up
Philip Morris International said yesterday that it had stopped cigarette production at the Aspropyrgos factory of its Greek affiliate, Papastratos, which was now exclusively producing HEETS, the tobacco units used with its heated-tobacco product, IQOS.
‘This first full conversion of a cigarette factory is a landmark step in our vision of a smoke-free future where people who smoke switch from the most harmful form of nicotine consumption – cigarettes – to scientifically substantiated smoke-free alternatives,’ the company said in a note posted on its website.
‘The €300 million investment included the construction of three new buildings and the replacement of cigarette production lines with high-tech facilities capable of producing 10,000 smoke-free tobacco units per minute.
‘The conversion of the factory started in August 2017. The facility is expected to be fully operational by the end of 2018 and will create 400 new jobs.’
“This is a historic day for our company,” said André Calantzopoulos, PMI’s CEO. “Papastratos is the first of our factories to end cigarette production and fully shift to manufacturing our smoke-free alternatives.
“We will continue to convert existing sites and invest in new facilities to answer global adult smoker demand for better alternatives to cigarettes.
“We made a commitment to provide all people who would otherwise continue smoking with potentially less harmful products. The momentum around this revolutionary change for the benefit of the world’s 1.1 billion smokers, public health and society at large is growing, and we will continue working towards a smoke-free future.”
Along with PMI’s plant near Bologna, Italy, Papastratos is its second facility fully dedicated to manufacturing smoke-free products. PMI has announced plans also to transform, either fully or partially, its cigarette factories in Korea, Romania and Russia.
‘Since 2008, we have invested more than US$4.5 billion in scientific research, product and commercial development, and production capacity related to IQOS and other smoke-free products,’ the note said. ‘In 2017, over 70 percent of our global R&D expenditure and over 30 percent of our global commercial expenditure was allocated to smoke-free products.
‘We estimate that at the end of January 2018, nearly five million adult consumers around the world have already stopped smoking and switched to IQOS. Our ambition is that all those who would otherwise continue smoking abandon cigarettes and switch completely to scientifically substantiated smoke-free products as soon as possible. Appropriate regulatory policies and decisions can substantially accelerate the speed and magnitude of this historic change.’
Road to recycling
Australian researchers may have come up with a way of utilising discarded cigarette butts, according to a story by Ben Renner for studyfinds. org.
Researchers at RMIT (Royal Melbourne Institute of Technology) university have tested a mixture of asphalt and discarded cigarette butts, and the results so far are said to have been encouraging.
“I have been trying for many years to find sustainable and practical methods for solving the problem of cigarette butt pollution,” Dr. Abbas Mohajerani, the leader of the study and senior lecturer at RMIT, said in a release.
Mohajerani earned worldwide praise in 2016 after discovering a way to use cigarette butts in the production of bricks.
The researchers found that not only does the asphalt pass heavy traffic tests, it also reduces thermal conductivity.
The results of Mohajerani’s study were published in the journal Construction and Building Materials.
The studyfinds.org story is at: https://www.studyfinds.org/cigarette-butts-recycled-ground-asphalt/.
New IQOS store in London
Philip Morris Limited is scheduled today to launch its fourth IQOS store in London, UK – this one in High Street Kensington.
The new store will offer adult smokers, across 800 sq ft of retail space, seven days a week, the opportunity to have guided trials by trained IQOS staff to learn about how the heated tobacco product works, and to discuss how they can switch from smoking cigarettes to IQOS.
The store will include, too, an interactive educational display to provide more information on heated tobacco technology and the IQOS product.
Customers will be able to acquire personalised and embossed IQOS devices and accessories.
The new store, which was designed by iD, uses white, grey- and copper-toned design elements to retain and accentuate the original features of the building, which was previously occupied by a large bank.
The windows of the store use a mixture of digital and feature displays, in neutral tones, to accent the stone facade of the building.
“This is a significant development in IQOS’s commercial expansion in the UK and we are delighted to be bringing the unique, retail experience that our IQOS stores deliver to High Street Kensington,” said Peter Nixon, MD of Philip Morris Limited UK & Ireland. “As a company, PMI has never previously had retail stores in the UK and so the opening of our fourth store marks an important moment for us and our ambition for a smoke free future.”
In a press note, PM said the new store marked a further development in the pledge to convert 100,000 UK adult smokers to its heated tobacco product, IQOS, and to reach its goal of a smoke free future for the UK.
‘This pledge is part of Philip Morris International’s … global commitment to offer adult smokers a range of alternative smoke free products for those who continue to smoke…’
London’s first IQOS store opened on Wardour Street, Soho, in December 2016, while the second and third stores opened in Westfield and Boxpark Shoreditch in September last year.
Canada in dialogue
The Global Forum on Nicotine (GFN) is due to stage a free-to-attend, tobacco-harm-reduction dialogue in Vancouver, Canada, in April.
The dialogue, Tobacco harm reduction: different strokes for different folks, or a consistent approach?, is to be held in partnership with the BC Centre for Disease Control and the Canadian Drug Policy Coalition.
It will be held from 09.00 to 16.30 on April 9, at the Morris J Wosk Centre for Dialogue in Vancouver.
‘The huge growth in the availability of safer nicotine products, with new technologies, such as vaping and heat not burn, as well as oral tobacco products, such as snus, has created greater opportunities for smokers to switch from a proven dangerous and unhealthy way to consume nicotine to much safer methods, according to a press note from the GFN, which has previously run series of dialogues in the UK and Ireland.
‘The emerging science surrounding both the technology and the products is positive and encouraging.
‘Vancouver has a proud tradition for supporting harm reduction for illicit drug use, including pioneering supervised consumption rooms for injecting drug use. The principles of harm reduction are well understood and have been enacted for many years with positive results.’
Participants will be addressed by international and local presenters, including:- Dr. Mark Tyndall, executive medical director, BC Centre for Disease Control;
- Professor Marjorie MacDonald, School of Nursing, University of Victoria;
- Professor Gerry Stimson, professor emeritus, Imperial College, London;
- Jacques Le Houezec, independent consultant in public health and tobacco dependence, France;
The presenters are due to examine:
- The history of harm reduction in Vancouver, the lessons learnt and the implications for this approach in relation to tobacco and smoking.
- Tobacco harm reduction as the ‘new kid on the block’ and what the emerging evidence is telling us.
- What does regulation look like and what are the elements that make for appropriate and effective regulation?
- The consumer experience – what products do people use and what are the results for them?
- What are the key issues for policy-makers and how can we ensure buy-in from all stakeholders?
Attendance at the dialogues is free, but participants are required to register at: https://gfn.net.co/dialogues/register.
More details about the dialogue are at: https://gfn.net.co/dialogues/vancouver-2018.
Vaping forum announced
The UK Vaping Industry Association (UKVIA) intends to stage its first industry forum next year.
The Going for Growth Forum will focus on ‘building upon the dramatic growth in the sector and realising predictions of a market value of more than £4bn by 2021,’ according to a UKVIA press note.
The event, which is scheduled to be held on April 23 at the Kings Fund, a leading think tank in health and social care, is expected to bring together more than 200 industry representatives, parliamentarians, public health professionals and researchers.
“These are exciting times for the industry as more organisations acknowledge the vital role that vaping plays to help smokers quit, including the government, Public Health England and the British Medical Association,” said UKVIA board member John Dunne.
‘Such progress is matched within the industry, with predictions of a record Christmas for trading, the rise in retail vape shops across the country and the UKVIA membership doubling in the last six months.
‘Now the UKVIA is firmly established and the sector is at an important stage in its ongoing development, it is the perfect time to stage our very first industry forum.
‘Ultimately, we want to establish the biggest business to business event in the sector.’
More information is at: http://www.ukvia.co.uk/2018-forum.

FDA calls iQOS meeting
The US Food and Drug Administration has published a Federal Register notice announcing a meeting of the Tobacco Products Scientific Advisory Committee (TPSAC) to discuss modified risk tobacco product (MRTP) applications submitted by Philip Morris Products (PMP).
The meeting, which is scheduled for January 24-25 at the FDA’s White Oak campus, was the subject of a Center for Tobacco Products (CTP) press note.
The committee is due to discuss scientific issues related to the MRTP applications submitted by PMP for its iQOS system and several Marlboro HeatSticks products, which are currently under scientific review by the FDA.
Requests for the presentation of oral comments at the TPSAC meeting have to be submitted by December 27.
Written comments have to be submitted by January 4.
More information is available at: https://www.federalregister.gov/documents/2017/11/09/2017-24379/tobacco-products-scientific-advisory-committee-notice-of-meeting

Altria to hold Investor Day
The US-based Altria Group is due to host a webcast of its Investor Day in Richmond, Virginia, on November 2.
The presentation will be live from about 10.20 through 12.20 Eastern Time, resuming about 14.30 following lunch and a manufacturing-center tour. The webcast is scheduled to end about 15.00.
The webcast, which will be in listen-only mode, will feature presentations by the company’s chairman, CEO and president, Marty Barrington, and other members of its senior management team.
Pre-event registration is necessary at www.altria.com/webcasts.
An archived copy of the webcast will be made available on altria.com or through the Altria Investor App. The free app is available for download at www.altria.com/irapp or through the Apple App Store or Google Play.

IQOS pops up in London
The launch of a third IQOS store in London, England, marks a further development in Philip Morris’ pledge to convert 100,000 UK smokers to this heated tobacco product, and to reach its goal of a smoke free future for the UK, according to a press note issued by PM Limited UK & Ireland.
Yesterday, the company opened an IQOS store in Shoreditch, London, at Boxpark, which is said to be the world’s first pop-up mall.
This follows the launch of the brand’s first UK store in Soho, London, which opened in December, and its second store, which opened in Westfield, London, earlier this month.
PM said the new store was Boxpark’s first vaping pop up, and that its opening reflected the increasing numbers of people looking for alternatives to traditional cigarettes.
‘The new East London store marks a further development in the brand’s pledge to convert 100,000 UK smokers to its heated tobacco product, IQOS, and to reach its goal of a smoke free future for the UK, where cigarettes are no longer sold,’ the company said.
‘This pledge is part of PMI’s [Philip Morris International’s] global commitment to offer smokers a range of alternative smoke free products for those that cannot completely quit (which is always the best option). Building its future on this vision, PMI has invested over £3bn into research to develop a broad portfolio of potentially reduced risk products, which includes IQOS and its unique heat not burn technology.
‘The Boxpark IQOS store, which will be open seven days a week, will offer a bespoke in-store experience for customers. In the store, adult smokers will be given guided trials by trained IQOS staff to educate them about how the heated tobacco product works and discuss how they can switch to IQOS. Customers will also have the chance to customise their IQOS device at the store’s personalisation station, which will be a unique offering for customers visiting the Boxpark store.
‘Designed by international creative agency Avantgarde, the Boxpark store is minimalistic with an all-white interior and a lightly coloured stone floor. Intelligent design details such as mirrors, hidden drawers and back lighting create space within the store for a sleek and modern finish.’
“This is a significant development in IQOS’s retail expansion in the UK and we are delighted that the Boxpark team has recognised the unique retail experience our IQOS store will bring,” said Peter Nixon, MD of Philip Morris Limited UK & Ireland.
“Our first store which launched in December of last year has seen a significant response from customers and we are thrilled to be bringing IQOS to East London as part of our growing retail expansion.”

CORESTA deadlines near
The CORESTA Secretariat says that the deadlines for online registration for two Joint Study Group Meetings are ‘fast approaching’.
The Smoke Science and Product Technology (SSPT2017) meeting is due to be held at Kitzbühel, Austria, on October 8-12.
The SSPT2017 website is at www.sspt2017.org, where the online registration deadline is September 27.
And the Agronomy & Leaf Integrity and Phytopathology & Genetics meeting (AP2017) is scheduled to be held at Santa Cruz do Sul, Brazil, on October 22-26.
The AP2017 website is at www.corestabrazil.com, where the online registration deadline is October 8.