22nd Century Group will significantly expand its growing program for VLN reduced nicotine content tobacco based on the company’s latest sales projections. This new planting for VLN tobacco is in addition to the company’s sizeable inventory of VLN tobacco, which is earmarked for the launch and initial sales of 22nd Century’s VLN reduced nicotine content cigarettes.
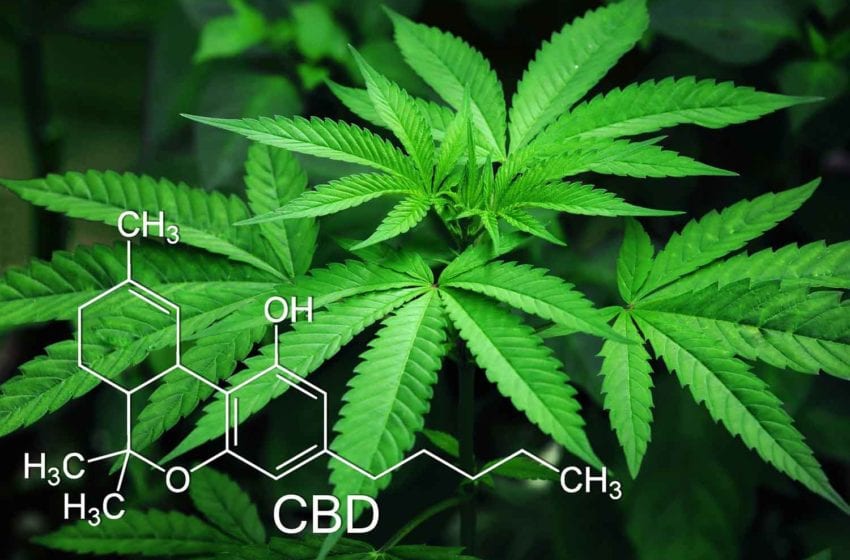
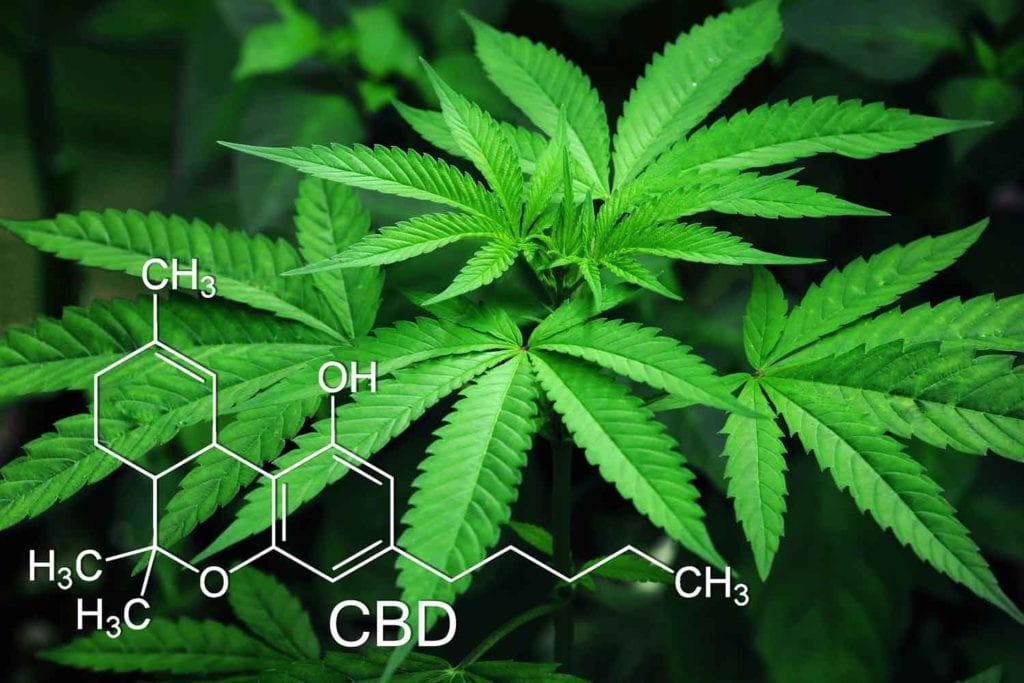
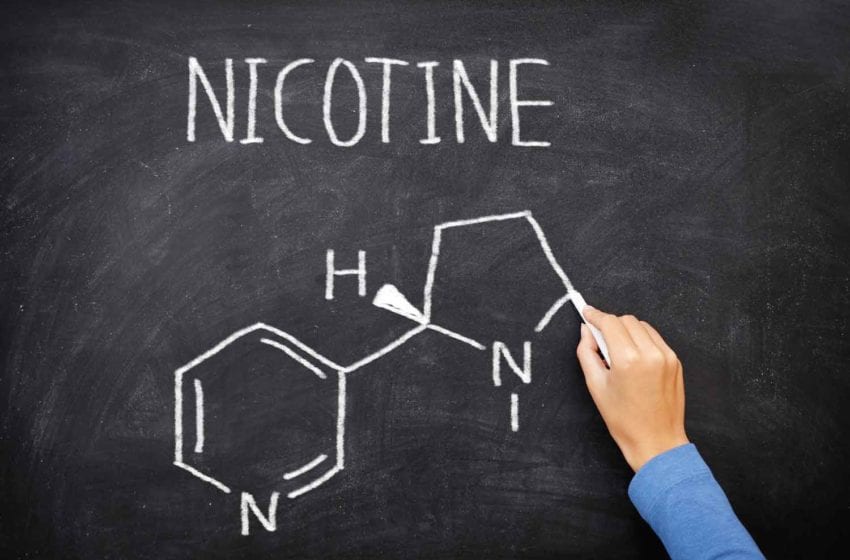
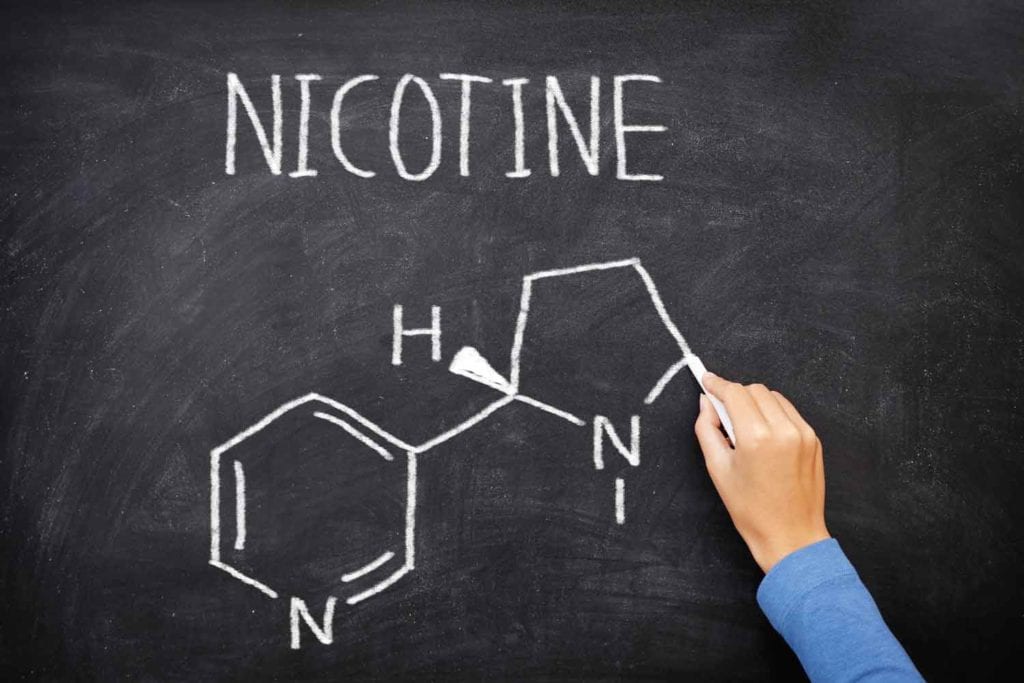
22nd Century’s Modified Risk Tobacco Product (MRTP) application for VLN cigarettes is currently in the final stage of review with the U.S. Food and Drug Administration (FDA). Once authorization is granted, 22nd Century will begin marketing its VLN cigarettes, which contain 95 percent less nicotine than conventional cigarette brands.
“We are prepared to launch our VLN cigarettes within 90 days after receiving marketing authorization from the FDA,” said James A. Mish, chief executive officer of 22nd Century Group, in a statement. “There are more than 34 million smokers in the United States and research shows that a majority of these smokers are looking for alternatives.”
22nd Century says it is also interested in licensing its technology other cigarette manufacturers to help them comply with the FDA’s plan to make all cigarettes non-addictive. “We look forward to the tobacco industry joining our efforts to truly reduce the harm caused by smoking and protect future generations from ever becoming addicted to cigarettes,” said Mish.
In partnership with select tobacco farmers, 22nd Century will plant this new VLN tobacco throughout the U.S. tobacco belt. The company’s proprietary, reduced nicotine content tobacco contains, on average, just 0.5 milligrams of nicotine per gram of tobacco, compared with conventional cigarette tobaccos which often contain 20 mg to 30 mg nicotine per gram of tobacco.
Published in 2017, the FDA’s comprehensive plan for tobacco and nicotine regulation aims to set a product standard for cigarettes that achieves “minimally or non-addictive” levels of nicotine. The FDA projects that within the first year of implementing a mandate, it will help more than five million adult smokers to quit smoking and will save more than eight million American lives by the end of the century.