Canada’s proposed regulations would make e-cigarettes more harmful, warns ITCAN. Read More
Tags :Canada
The product were sold without market authorization, according to the agency. Read More
Canada’s new health minister is breathing new life into a 2021 proposal to ban vape flavors nationwide.Read More
Imperial questions why activists who called for a flavor ban are now silent on illegal sales.Read More
Vaping activists warn that the measure could achieve the opposite of its objectives. Read More
Holland plans to restrict access to Zonnic as well as restrict flavors and marketing. Read More
Imperial Canada insists its products are intended for adult smokers who wish to quit. Read More
Imperial Tobacco Canada says the province's move will make it harder for smokers to quit. Read More
Imperial Canada says flavored e-cigs remain available three months after a ban took effect. Read More
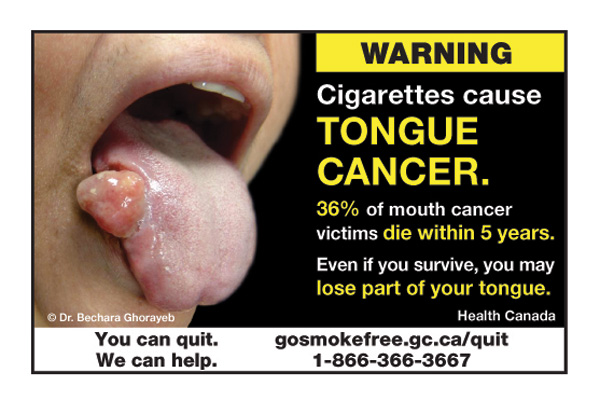
The government wants to prevent its images from losing their shock value. Read More