From a public health perspective, the misperceptions and misunderstandings surrounding nicotine are incredibly frustrating, according to David Sweanor, adjunct professor of law at the University of Ottawa, who moderated the GTNF panel on the public perception of nicotine. Science has proven that it is the delivery system [combustible cigarettes], not the nicotine itself, that causes the deadly diseases suffered by cigarette smokers.
Sweanor said Sweden was an excellent example of a country where the use of different delivery systems, such as snus, led to massive decreases in the number of combustible cigarette smokers. Sweanor said it would be a major benefit to public health if the industry would or even could do more to educate consumers, public health groups and legislators about the facts. He emphasized that people can only make as good a decision as the information available to them allows.
“We already knew from the work of Michael Russell that people smoked to get nicotine, but they died from the smoke. Nicotine wasn’t the problem. It was the delivery system that was the problem,” Sweanor explained. “We’ve known that for 50 years. And we’ve seen examples from places like Sweden where people can move to an alternative product and have disease rates that are massively lower than what you see elsewhere. In fact, looking at the long-term users of a product called snus, it is very hard to find anything to distinguish their disease risks from those people who don’t use any tobacco or nicotine product at all.”
The first speaker on Sweanor’s panel was Carolyn Beaumont, a general practitioner, educator and founder of SmokerHealth Telehealth and Medical Nicotine Scripts who for the last three years has been prescribing vaping products to cigarette smokers in Australia. She said that there is an outpouring of need and frustration and even fear from the smokers that she works with. Australia’s vaping rules permit vaping products only through prescription and severely restrict the products that can be prescribed. Beaumont presented several quotes from former smokers showing how vaping had changed their lives for the better.
“They really want their stories to be heard …. Smokers want to be heard—not judged—supported and advocated for. They’re also very fearful that if they can’t get their vape, they will return to smoking, and that seems true as well,” said Beaumont. “For those of you who are not sure how successful vaping is in [supporting] smoking cessation—it’s very effective.”
The next speaker, Delon Human, president of Health Diplomats, said that misperceptions surrounding nicotine were causing people to die. “At the heart of nicotine misperception lies an issue that we are wasting unnecessary lives,” Human told attendees. “We are allowing the misperception of nicotine to lead to disease. And that is a time that we absolutely have to take hold of the stakeholders, who can change those perceptions.”
He stated that the World Health Organization’s failure to differentiate between tobacco and nicotine, and between combustibles and noncombustibles, has caused the spread of misinformation among the government and nongovernment organizations it influences. “If you read WHO documentation … on the one hand, nicotine is part of the WHO list of essential medicines. Nicotine as part of nicotine-replacement therapy as prescribed by physicians and health professionals for smoking cessation,” said Human. “And on the other hand, there’s an all-out war on nicotine. What has happened over the years, over the last 50 years, is that the so-called war on tobacco has changed into the war on nicotine.”
Human also noted that there is a serious amount of misperception surrounding nicotine among physicians and consumers. He mentioned a recent study from the Foundation for a Smoke-Free World that found that on average, nearly 77 percent of doctors mistakenly believe nicotine causes lung cancer, and 78 percent believe it causes atherosclerosis. While on average 87 percent of doctors at least moderately agree that helping patients quit smoking is a priority, lack of training and nicotine knowledge adversely impacts quitting and harm reduction advice, according to the study.
“It found that 58 percent of those respondents thought that [nicotine] caused cardiovascular or heart disease, which again shows you that the level of misconception is not only dangerous—it’s sick …. It’s 2023 and so simple of a situation, but suddenly physicians have a complete misconception of what nicotine is,” he said. “In our own study in five countries, we [found that among] GPs [general practitioners], there was a persistent belief that nicotine is the most awful aspect of smoking. Nicotine causes cancer, and nicotine causes heart and lung disease.”
Human pointed out the positive outcomes of correcting misconceptions about nicotine, including improved health for smokers and lower smoking rates. He proposed that medical professionals should receive new training on the effects of nicotine and the advantages of tobacco harm reduction. Human said that companies should also refrain from marketing to youth and keep up with the research and development of new reduced-harm products.
“In terms of physicians, what can they do for misperceptions to be corrected? No. 1, training needs to be updated to the 21st century. Doctors need to know that nicotine does not cause cancer. It’s a crime for doctors to think that nicotine causes cancer or heart disease or lung disease in a way that they perceive it now, so correct the training,” he said. “No. 2, make sure that doctors understand what harm reduction is. Harm reduction is really part of everyday medical life. That’s what we do in medical practice. You’re trying to reduce the harm.”
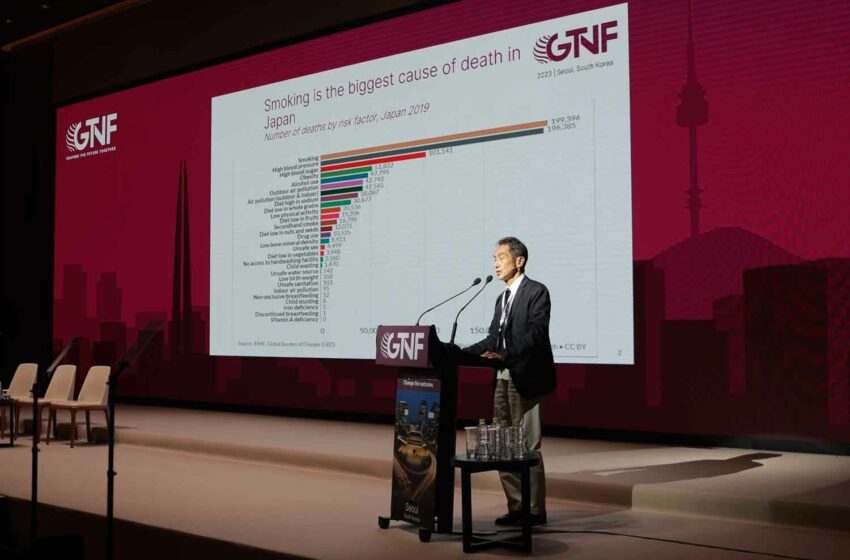
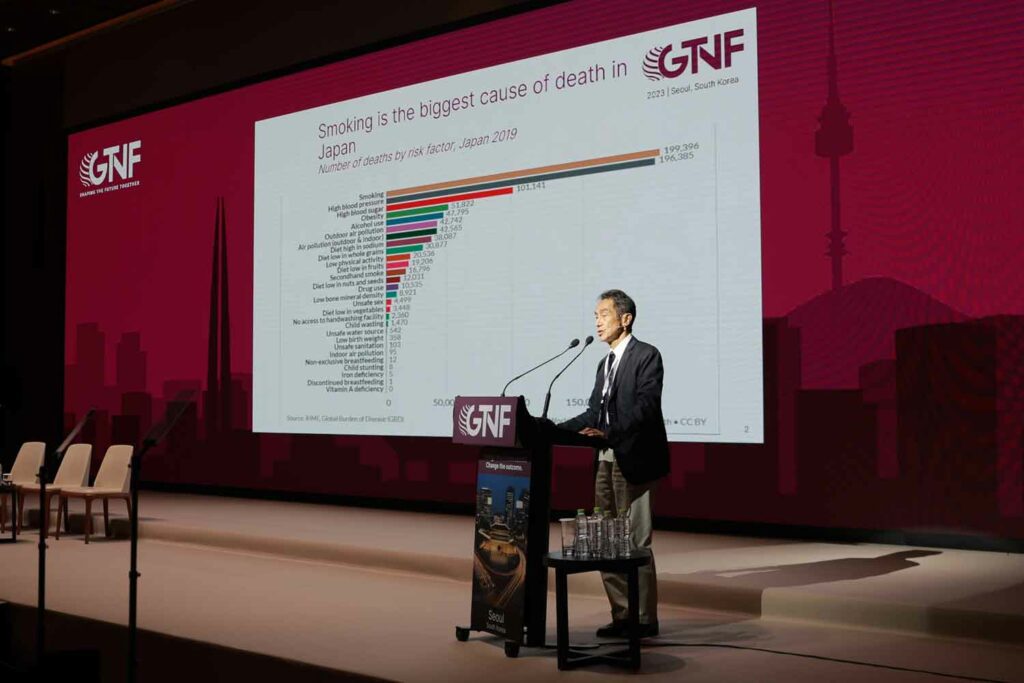
Hiroya Kumamaru, a cardiovascular surgeon and vice director of AOI International Hospital in Japan, said that in his country, there is also a wide misperception among physicians that nicotine is harmful. He argued that the industry needs to think about how best to educate regulators on understanding the effects of nicotine and the risks of different delivery systems. “Many, many … GPs are thinking that nicotine is quite harmful itself [in Japan], and we have to educate them somehow. Thinking about how we can [address] this issue, I tried to have a small meeting in the Swedish embassy about four years ago to educate not only physicians but also media and governmental officers to understand the concept of harm reduction in tobacco, in smoking,” explained Kumamaru. “But it was still difficult because some of the physicians, even [ones that] were working in a university hospital or working in the Ministry of Health, were saying that smoking is a sin …. They didn’t care about the difference [between] vapor and cigarette smoking. Because they say nicotine drug dependency is a very bad agent. We have to think once more to educate these people to understand.”
Kumamaru said Japan has seen a historic decline in the number of combustible smokers because of the rise of heated-tobacco products. He said more than half of the combustible market has disappeared in just a few years. He also agreed that the industry could accomplish more if more were done to battle misinformation.
“We’re still stuck with this problem of it. People and regulators can only make as good a decision as the information available to them allows us. And people believe that using nicotine is about sin rather than about health. If people don’t understand that [combustible cigarettes, not] nicotine causes cancer, what can you do? …. Millions of Americans who are able to move between the [various harm reduction] products never had the information that one product is very likely to kill you and [that] the alternative product is massively less hazardous.”
Mohamadi Sarkar, a fellow of scientific strategy and analysis and regulatory affairs at Altria Client Services, told attendees that the science on nicotine is not new. He said that even though many mistakenly believe that vaping is just as dangerous or even more so than smoking combustible cigarettes, there is a plethora of evidence to show that vaping contains fewer harmful chemicals than cigarette smoke.
“We often hear that ‘Well, these products have not been on the market long enough, so there is no long-term epidemiology.’ We don’t need it. What we do know is that cigarettes have 70,000 chemicals. Seventy of them are carcinogens and linear, cardiovascular and respiratory toxins,” he explained. “On the other hand, smoke-free products like e-vapor or [heated-tobacco products] are nicotine-positive and have far fewer chemicals.”
In the end, all the speakers agreed that the industry could do more to battle the misinformation surrounding reduced-risk products. The vaping industry needs a unified voice. “We need unified goals for all the stakeholders to communicate …. We know that education works. Education has changed perceptions,” said Sarkar. “We need immediate action.”
 In a video recording posted as part of the Bonus Content of the September Global Tobacco and Nicotine Forum in Seoul, South Korea, Robert Burton, group scientific and regulatory director of the Plxsur Group, made the case for the vaping industry to comply with the highest standards of integrity—even going beyond the requirements of regulations where necessary.
In a video recording posted as part of the Bonus Content of the September Global Tobacco and Nicotine Forum in Seoul, South Korea, Robert Burton, group scientific and regulatory director of the Plxsur Group, made the case for the vaping industry to comply with the highest standards of integrity—even going beyond the requirements of regulations where necessary.