Suppliers of instrumentation and lab services are focusing on novel nicotine products.
By Stefanie Rossel
Two things are for sure: Instrumentation and lab service suppliers don’t have any time to be idle. And a look at their most recent innovations conveys a good idea about where the nicotine industry is heading.
Two years ago, instrumentation manufacturers and providers of laboratory services were busy supporting makers of electronic nicotine-delivery systems (ENDS) with their submissions for premarket tobacco product applications (PMTAs) to the U.S. Food and Drug Administration.
“It certainly has been an interesting two years for ENDS manufacturers,” says Chris Allen, chief executive officer of Broughton, a U.K.-based contract research organization helping companies with delivering full-service regulatory projects.

“In the last few weeks, there have been marketing denial orders (MDOs) for multiple Myblu and Juul products as well as three high-profile manufacturers being awarded marketing orders for their products. Broughton is thrilled to have played a significant part in the granting of some of these marketing orders, and we expect more to come soon.
“With these five separate PMTA decisions, the FDA has given the industry an indication of where the bar is set for gaining a marketing order. It also gives additional insight into its evaluation process as the rationale behind the Myblu and Juul MDOs were very different. Although not everyone benefited from these decisions directly, they have given manufacturers new confidence to move forward with product development and future business roadmap decisions.”
Nicotine companies are now considering new PMTA applications, modified-risk tobacco product (MRTP) applications and marketing authorization applications for products under European Medicines Agency regulation. “The industry isn’t losing its appetite or ambition for innovation and new product development,” says Allen. The PMTA process is now firmly established as one of the costs of selling next-generation nicotine products in the U.S., and manufacturers have adapted to this and are moving forward.”
In addition to full-service solutions, Allen observes a significant interest in Broughton’s standalone services, such as toxicological assessments and laboratory services, many of which are in support of preparing for or responding to PMTA deficiencies. “Unfortunately, many companies have been provided with a substandard service for their PMTAs. Now [that] we have understood the bar for gaining approval, many companies are requesting us to provide extra evidence to submit before their applications enter into substantive review.”
Focus on Reduced-Risk Products

Reduced-risk product (RRP) testing continues to be at the core of instrumentation suppliers’ business. “The industry as well as governmental organizations still have a strong focus on new-generation products like e-cigarettes and heated-tobacco products (HTPs),” notes Joost Elvers, group leader of key account management at Borgwaldt KC, a German manufacturer of quality control instruments and devices that is part of the Hauni group of companies, which also includes metrology specialist Sodim. “New product designs combined with upcoming further regulations and standardizations will continuously require close support,” says Joost. “Besides, the combustible product category experienced a focus revival with the opening of markets to cannabis and hemp products. We are therefore strengthening our portfolio of quality control equipment for the different product categories as well as our broad range of emissions testing devices. Furthermore, with the reduction of Covid-19 measures in companies and countries, our team of service engineers has increased its service activities again to support our customers on site in addition to the remote services that have been introduced over the last 2.5 years.”
U.K.-based Cerulean is focusing on three tobacco-related areas, according to Ian Tindall, head of innovation and marketing.
“The first is supporting companies within the ever-expanding heated-tobacco product market,” he says. “This still requires a lot of specialist equipment to generate information needed in support of MRTP applications as well as other product development activities.
“Also, with increasing amounts of products coming to full-scale production, we are finding routine quality assurance equipment is definitely an area we see as expanding. Partly, we are addressing this need by working with our sister company G.D in providing closed-loop control for makers and combiners and partly, it is rolling out and deploying our X-ray equipment to monitor combiner output.
“A second area we are really excited about is in producing routine test equipment for modern oral products as we see this as a rapidly growing area where quality assurance can be automated and improved. We launched a product, the Orion, just for this market, and we have received almost overwhelming positive feedback from companies.
“The final area is in supplying test equipment for the safe regulation of legal recreational cannabis use in the United States. We have rapidly found that this is not another cigarette-type application, and we are learning alongside clients how to ensure the safety and compliance to regulation of these now legal products.”
While the Orion is currently one of Cerulean’s most sought-after products, Tindall has detected another trend, which he finds difficult to describe. “It’s the service that starts with a customer saying, ‘I want to measure something, but I am not quite sure what,’” says Tindall. “The service is about working with customers to develop test and measurement equipment for new-to-the-world products that have no background of tests to ensure conformance.”
While Cerulean’s commitment to customer confidentiality prevents Tindall from elaborating on current projects, he cites the example of a customer who wants to prevent burst capsules from wetting tipping paper. “We might come up with a way to measure the radial and longitudinal positioning of the capsule in the filter, which prevents liquid getting too close to the outside tipping paper,” he says.
New Testing for New Products

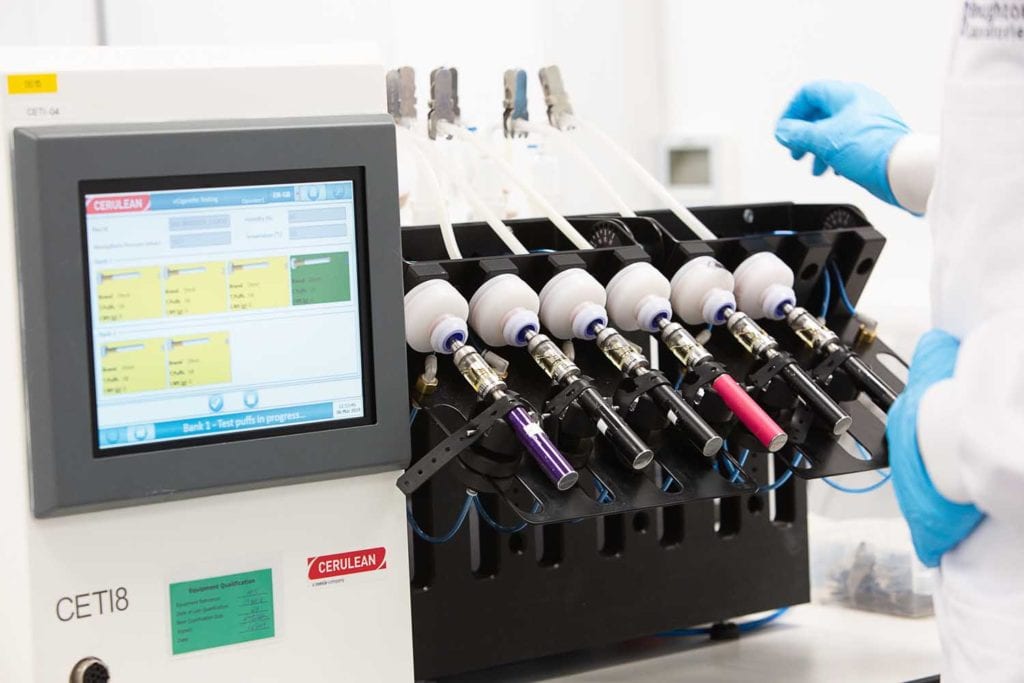
Cerulean’s Orion, the first automated snus test station to enter the market, is but one example of an array of innovations for testing novel nicotine products. Currently, the Orion measures the weight, length and width of the pouch along with the tensile strength of the pouch seams as well as extension against load.
“We will, before the end of Q3, deploy extra measurements in the form of longitudinal pouch seam position and overlap, pouch transverse seam size and pouch moisture,” says Tindall. “We are listening to the customer base and expect to be adding further enhancements in the future once we have really established what is important to our customers, including the potential for auto-sampling and feedback to a maker to reduce reject rates. We expect Orion to follow the trajectory of most of our products in that it will be developed and enhanced as our customers’ needs change.” The Orion can be used for all types of modern oral pouches as long as the size fits in the maximum and minimum dimensions allowed and the pouches follow a rectilinear format.
Sodim recently introduced a test station dedicated to the testing of HTP consumables. “HTP and RRP confront us with many challenges, such as different format compatibility and new measurement request,” says Christine Camilleri, director of sales and marketing at Sodim.
“This, combined with sustainability, guides our development team toward instruments [that are] fully scalable, responding to the needs of this market as regards to quick product changes. All our test stations are compatible with HTP products of any size. Multiple diameter measurements on filter rods are an example.”
From Borgwaldt comes the LM1E DtL, a new vaping machine that provides direct-to-lung testing. ISO 20768 requires aerosol to fill the mouth before entering the lung, which is commonly named mouth-to-lung vaping.
Consumers, however, tend to vape different products differently. Borgwaldt KC developed the LM1E-DtL based on the draft development standard of CEN/TC437. “This vaping machine fulfills the requirements of an additional vaping regimen considering inhalation from an electronic cigarette directly into the lung,” explains Elvers. “As you can easily imagine, the emissions composition differs from that generated under the ISO 20768 process and therefore reflects the consumer exposition much better.”
Design Support
The industry’s focus on RRPs is reflected in the demand for lab services too. With nicotine pouches, one of the most rapidly growing segments within the reduced-risk category, Broughton is seeing much interest in its consulting and testing services. “There are some interesting innovations around oral pouch materials, so our feeling is that the scope for oral pouches will grow beyond nicotine. The products are covered by the PMTA process within the U.S., so we have been busy providing support for these applications. Within the EU, any work performed is typically to support product development and/or due diligence. As per the ENDS analysis, the focus is placed upon nicotine content and HPHCS [harmful and potentially harmful constituents]. However, automated (flow-through) dissolution analysis is widely used to support the R&D process.”
Broughton is also offering development services for next-generation products as well as for cannabinoids, another big theme in the sector. The company already has a medicinal cannabis and CDB business. For novel nicotine products, it has launched a division that helps customers design their products in a way that increases the chance of regulatory approval, for example by ensuring that development decisions taken early in a project support the later stages of a planned regulatory submission or go-to-market strategy.
“This could be early development material or ingredient selection to expedite extractables and leachable studies or ensuring product designs are suitable for mass-market manufacturing scale-up,” says Allen. “Our services are completely scalable to the needs of the client so we can help with one stage of product realization or work as an extension of an in-house development team all through the product lifecycle. We created the service in response to requests from existing clients, so we know there is a demand for this sort of expert advice and consultancy.”
As far as trends are concerned, Allen sees growth in the diversity of nicotine-delivery systems. “There are more heated-tobacco products, more modern oral nicotine pouch manufacturers plus innovations like water-based vape devices and new heating mechanisms,” he says. “Disposable vape devices are also growing in popularity, and there are some exciting innovations around device material selection, especially focused on improving the recyclability of products, which we predict will be very important in the future. At Broughton, we work with a wide variety of ENDS manufacturers of different sizes and backgrounds. We are seeing a lot of new technology coming from regions like the Middle East, India and Indonesia in addition to where you’d expect it to come from, such as the USA and China. It really is a very dynamic industry with lots of new players looking to bring something different and differentiated to the market.”
Greener Measurements
While flexibility plays an important role in novel-products testing equipment, Borgwaldt KC and Sodim have also noticed growing demand for sustainability. “We can currently see two trends gaining momentum within our customer base. One is for sure the change in available product portfolios of some of our customer groups; the other is the realization of sustainability targets in the instruments environment,” explains Elvers.
“We therefore spend many efforts in making flexible emissions testing solutions for combustible cigarettes and cigars as well as for the new electronic product segment of ENDS. The successful launch of the 10-port vaping machine NGX10 and its continuous modularization with further add-ons shows us the high demand for such a modern and ENDS-dedicated solution on the market. Besides this, the trend of rethinking life cycles of instruments and how they can be converted for new demands to save resources made us create our ‘lifetime extension’ program in which we update older instruments with the newest measuring technology by fully building upon existing infrastructure and reusing or refurbishing existing parts for a more sustainable outcome.”
Camilleri notes that customers are moving to “green” products, such as hemp and cannabis. “On physical parameters, they are aiming to get fast measuring solutions in a quickly changing market,” she says. “Specific developments become the norm compared to standard solutions in the past. We orientate our products on super flexible instruments adapted to different market environments and production allowing long-term evolution of test stations, including the possibility to upgrade them to cater to new product trends. Our products can have several lives in different segments of the industry, reducing the impact on the environment.”

Testing Without Standards
As more countries legalize cannabis, instrumentation makers detect new opportunities—even though testing standards are not yet in place. “Weight is currently the most important parameter, but we also see a new interest to measure the same physical parameters as in conventional cigarettes to improve the quality of the products and reduce cost generated by waste,” says Camilleri, whose company has adapted its Sodiline and Sodiqube test stations to cannabis testing.
Borgwaldt and Sodim are active in the raw material and emissions testing segments of cannabis products. “Combined with the experience gained with production machines of our sister companies Garbuio and Hauni, we established ourselves as a main contact point for raw material and production control as well as emissions testing for cannabis products,” says Elvers.
Borgwaldt has developed an electrostatic precipitation trap, HV1, which is used to trap emissions for the analysis of metals. Being a phytoextracting plant, cannabis collects and saves metals from the soil so that these elements will be released during the smoking and consumption process. “This smoking and vaping machine-independent solution can be used as a flexible add-on for the emission control of metals of cannabis products,” says Elvers.
Cerulean also has an electrostatic precipitator trap upgrade kit in its portfolio. The company has been working with Kaycha Labs in Denver, Colorado, USA, to overcome some of the problems inherent in the Colorado state requirement for emissions testing for metals, and it has published several white papers describing some of the changes required to allow a vaping machine to work with highly viscous cannabis oils. “This work supports the multiple machines we have sold to legal operations in the United States and hopefully demonstrates our commitment to this new industry,” says Tindall. He hopes that a set of practical conditions and analysis standard operating procedures can be adopted by the cannabis industry that provide a basis for comparisons from state to state.
Agility is Key
Regarding future requirements for testing equipment and lab services, Sodim and Borgwaldt KC say agility will be key. “Demands change within months, [and] products appear and disappear on the markets in a short period of time, therefore we as a supplier of quality control and emissions testing equipment have to keep up and even overtake these market demands and show our ability to react fastest to new market challenges,” says Camilleri, speaking for both companies.
“Also, digital solutions within the instruments business are expected to play a bigger role in the future. This can be related to the use of data being generated by measurement equipment or the combination and common usage of such data by the measuring instrument and the manufacturing systems. Even the partial replacement of physical measurements by a digital process is something to be considered as a future requirement.”
“My personal view is that we have probably hit, or are near to hitting, an innovation ceiling for vaping products,” predicts Tindall. “HTPs are still in a growth phase, and there will be other entrants beyond the big players currently in the market. There will be novel HTPs for sure over the coming five years.”
Tindall expects quality assessment and quality control for physical tests to transition from the laboratory to the production floor. “This means we need to have robustness and simplicity of operation in the forefront of our equipment design,” he says. “Especially if, as I expect, HTP manufacture is increasingly accompanied by more stringent traceability [requirements] as HTPs become even more highly regulated than combustible cigarettes. This will make current good manufacturing practice a baseline requirement that our equipment would need to support. Moreover, the interconnectedness of manufacturing processes and data retrieval becomes a fundamental of design and not just an afterthought.”
The recreational cannabis market, he says, will continue to spread. “This will mean that belatedly, there will be regulation of emissions, requiring new vaping equipment.”
With more than 1 billion adult smokers in the world—a number that is still increasing—Allen expects demand for testing to support regulatory submissions to increase over time, with a demand for more sensitive testing criteria, more in-depth analysis of test data and great insight from real-world evidence based on human factor studies over longer time periods.
“Remember, this is still a very young industry, so continuing to collect data is going to be essential to underpin belief in reduced-risk products and their contribution to tobacco harm reduction,” he says.