Even as cigarette dollar sales increase during the Covid-19 pandemic, IQOS expansion remains Altria’s primary focus.
By Timothy S. Donahue
Covid-19 has slowed the traditional decline of U.S. cigarette sales. With less opportunity to spend on travel and entertainment, consumers have had more money to purchase tobacco products, according to Billy Gifford, CEO of Altria Group. Since the start of pandemic lockdowns in mid-March, traditional cigarette sales have increased over the same period last year, breaking a longstanding trend.
During Altria’s second-quarter earnings call, Gifford said that the cigarette category has proved resilient during the pandemic. Based on year-to-date industry volume performance, the largest U.S. cigarette manufacturer has adjusted its estimated 2020 volume decline rate to a range of 2 percent to 3.5 percent, down from its previous estimate of 4 percent to 6 percent. The company also increased its annual dividend by 2.4 percent, saying it had more clarity on the pandemic’s effects on consumer demand. It is the company’s 51st consecutive annual dividend increase.
“Remember, last year, the cigarette category peaked its decline at 6 percent, then it receded to 5.5 percent in the third, and then down to 4.5 percent in the fourth,” Gifford said. “So a little bit tougher comparisons were also included in that forecast. But it’s a fluid environment and it’s something that we’ll continue to monitor.” Altria’s forecast was lower than its prior outlook, which was withdrawn due to the uncertainties around the pandemic’s economic impact.
Cigarette sales have seen a series of ebbs and flows through the first half of 2020. When some U.S. state governors began issuing stay-at-home orders in mid-March, combustible cigarette sales volume rose 1.1 percent for the week that ended March 22, according to Nielsen. Those sales were likely generated by consumer stockpiling, according to Gifford. For the four-week period that ended May 16, Nielsen reported only a 0.2 percent decline in sales volume for traditional cigarettes. Comparatively, sales volumes in 2019 fell 8.8 percent over the previous year (2018) in the four weeks to March 23, according to Nielsen data.


Bonnie Herzog, managing director at Goldman Sachs, stated in an email that the cigarette category is now holding steady. All channel cigarette dollar sales growth was up 3.1 percent for the two weeks ending on July 25. “Higher pricing more than offset a deceleration in cigarette volume, which was down 2 percent during the same time,” she said.
The pandemic is not the only factor driving increases in cigarette sales. Gifford says restrictions on e-cigarette flavors and the Sept. 9 deadline for premarket tobacco product applications (PMTA) to the U.S. Food and Drug Administration (FDA) have come together to create a perfect storm that is driving vapers back to combustible cigarettes.
Gifford told listeners that Marlboro’s second-quarter retail share for the overall cigarette category was 42.8 percent, down six-tenths versus the year-ago period. In April, Altria reported that older smokers who had switched to e-cigarettes were turning back to traditional cigarettes because of negative news coverage and regulatory crackdowns on vaping.
“As you’ll recall, earlier this year we noted an increase in the number of adult smokers aged 50-plus who moved from the e-vapor category back into cigarettes benefiting volumes from Marlboro and the cigarette category,” he said. “This demographic has a greater tendency to purchase discount brands than younger adult smokers, which increased the discount segment share at the start of the year. We believe the effect of this dynamic will have a lingering impact on Marlboro’s year-over-year retail share comparisons through 2020 … when you think about that, it’s a bit early on to tease out the exact impact from both of those, but that’s something that we’ll continue to monitor as we move forward.”


Photo: Altria Group
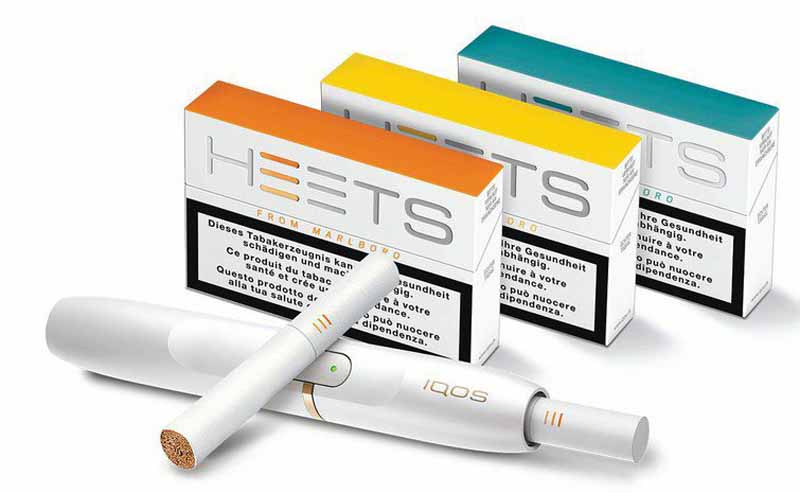
Looking toward the second half of 2020, Altria has high hopes for its IQOS heat-not-burn device. On July 7, the FDA issued exposure modification orders to IQOS. Gifford said he was pleased with the FDA authorization to market IQOS as a modified-risk tobacco product (MRTP) with a reduced-exposure claim. The FDA’s decision includes the device’s holder and charger as well as Marlboro HeatSticks, Marlboro Smooth Menthol HeatSticks and Marlboro Fresh Menthol HeatSticks.
IQOS is the first next-generation product to receive an MRTP. In a statement, the agency concluded that the available scientific evidence demonstrates that IQOS is expected to benefit the health of the population as a whole, taking into account both users of tobacco products and persons who do not currently use tobacco products.
According to the FDA website, a reduced-risk claim authorization would generally allow a company to say a product is less harmful than combustible cigarettes. However, according to the FDA, the current reduced-exposure claim authorization allows the manufacturer to only state that IQOS heats rather than burns tobacco and significantly reduces the production of harmful and potentially harmful chemicals. The decision follows a review of the extensive scientific evidence package Philip Morris International (PMI) submitted to the FDA in December 2016 to support its MRTP applications.
IQOS is produced by PMI and marketed in the U.S. by Philip Morris USA (PM USA), a subsidiary of Altria Group. Gifford said PM USA is making the necessary preparations to communicate the reduced-exposure claim to adult smokers, which includes developing new marketing assets and submitting them to the FDA in advance of being used.
“We’re looking forward to communicating with adult smokers the additional benefits of switching to IQOS. We’re excited to get back on track with our IQOS rollout and our future expansion plans to accelerate adult smoker conversion,” he said. “As many parts of the country began lifting restrictions in June, PM USA reopened the Atlanta and Richmond IQOS boutiques and just last week launched IQOS in its third lead market by opening a boutique in the SouthPark mall in Charlotte.”
Over the next 18 months, PM USA plans to launch IQOS in four new markets with large adult smoker populations and expand the availability of IQOS devices through retail partnerships, explained Gifford. PM USA also plans to expand its HeatStick distribution to the surrounding geographies in all seven IQOS markets. He said the commercialization approach for IQOS is designed to maximize the organic growth potential of the device by focusing first on the densely populated metro areas and then expanding outward as the user base grows.
“In Charlotte, PM USA launched a more disruptive retail fixture that communicates the benefits of real tobacco, no ash and less odor and expects to begin HeatStick distribution to retail stores in the next few weeks. By the end of August, we expect HeatSticks to be in a total of 700 retail stores across the three lead markets,” he said. “PM USA will continue to leverage its IQOS retail ecosystem, including IQOS mobile, pop-up and kiosk retail formats, which allows for more strategic and agile marketing plans. We’re making several digital enhancements to the IQOS website too.”
The IQOS website now includes virtual tutorials, and a new expert video chat functionality will be available this fall, according to Gifford. These digital enhancements and “the ability to have devices delivered to smokers in lead markets with the proper age verification” will provide smokers with a variety of options to “learn about and access IQOS,” said Gifford, adding that PM USA expects to use its “first-mover advantage” to expand IQOS responsibly.
“Our commercialization strategy is based on the learnings from our IQOS lead markets and PMI’s international results paired with our desire to continue avoiding use by unintended audiences. We believe that a sustained focus on the consumer journey from awareness to conversion is the key to achieving our vision,” Gifford said. “Word of mouth among IQOS users and their fellow adult smokers has been a critical factor to the global success of IQOS.”