As the success of iQOS continues, Philip Morris International gets ready to launch its other reduced-risk products.
By Stefanie Rossel

Has Philip Morris International (PMI) found the magic bullet the tobacco industry has long been searching for? The company’s first-quarter results suggest it is well on its way toward the smoke-free future that has become PMI’s declared goal.
Amid lower-than-expected cigarette shipment volumes, the company’s heat-not-burn (HnB) platform iQOS remained the shining light. PMI sold 173.6 billion cigarettes in the first quarter of 2017, 11.5 percent fewer than in the previous year’s quarter. However, sales of iQOS consumables, (commercialized as Heets and HeatSticks) ballooned to 4.4 billion units from 453 million units.
PMI’s HnB device continues to show impressive growth. The product also fits nicely with PMI’s broader strategy of divesting low- to no-margin volumes in favor of the premium end of its portfolio, says Wells Fargo Securities analyst Bonnie Herzog.
Japan, where iQOS was tested in 2014 and rolled out nationwide in 2016, remains the largest market for the reduced-risk product (RRP). Today, its performance continues to be the prime driver of PMI’s results in this market, according to chief financial officer Jacek Olczak.
The national market share of iQOS increased by 5.4 points in the first quarter to 7.1 percent, driven mainly by Marlboro Heets, which also helped grow the entire Marlboro share, including combustible cigarettes, to 17.1 percent. The performance is even more remarkable when viewed against the backdrop of industry volumes declining by 7.4 percent in Japan.
IQOS is also doing well in its other launch markets. In the first quarter of 2017, it reached national market shares of 0.5 percent in Italy, 0.9 percent in Switzerland and 0.4 percent in Portugal. In Germany, where iQOS was introduced in Berlin, Frankfurt, Wiesbaden and Munich last summer, the product reached a combined share of 0.8 percent in the last week of March, Olczak said.
The product is currently available in key cities in 24 markets. The most recent launches took place in Lithuania, Colombia, South Africa, Poland, Serbia and France.
New territory
That PMI is entering unchartered territory became obvious in several launch markets. In New Zealand, one of the most heavily regulated markets for tobacco products, the health ministry declared iQOS consumables—but not the device—illegal because they supposedly violated a 1990 law prohibiting tobacco products intended for uses other than smoking.
Arguing that the law was not developed for heated tobacco and e-cigarettes, PMI has continued marketing iQOS to registered adult smokers through an “invitation-only” website. At press time, the health ministry said it was still investigating the matter.
Taxation has been an issue, too. Russia, where iQOS has been test-marketed since 2015, has created a new tax category for heated tobacco products. Heets are now subject to an excise of rub4,800 ($82.1) per kilogram, according to ECigIntelligence. The rate is expected to rise to rub4,992 in 2018 and rub5,192 in 2019.
In Israel, two lawsuits accompanied the launch of the product. In March, the country’s health ministry announced that iQOS would be exempt from tobacco regulation until the U.S. Food and Drug Administration (FDA) decided on the company’s modified-risk tobacco product (MRTP) application. Given that the FDA’s assessment could take until 2018 or even longer, this implied that iQOS and Heets would be exempt from tobacco excise taxes, could be sold in Israel without health warnings and could be used in public for a considerable time.
Alleging favoritism, Israel’s domestic cigarette maker, Dubek, filed a lawsuit. The second petition, presented by third parties, including two health groups, asked for iQOS and Heets to be recognized as tobacco products. In early April, the justice ministry decided that, in the absence of an FDA decision, iQOS should be sold as a tobacco product, subjecting it to all restrictions on tobacco marketing, advertising and public smoking.
Seeking FDA approval
By the year’s end, PMI aims for iQOS to be present in 30 to 35 markets. High on the list is the United States, where the company has submitted not only an MRTP application but also a premarket tobacco product application (PMTA), which would allow PMI to market iQOS independent of the MRTP process. If the FDA approves the PMTA, the product would be marketed by Altria, the parent company of Philip Morris (PM) USA. Herzog predicts that iQOS will be launched in the U.S. this year.
Due to tax hikes, bans on tobacco marketing and smoking in public places, as well as growing health awareness, smoking in the U.S. has been declining since the 1960s. In 2015, prevalence stood at 15.1 percent. The figure is expected to further decline by around 3 percent annually until 2040, according to data published by the Centers for Disease Control and Prevention.
PMI is the first company to seek FDA approval to market an HnB product as being less harmful than traditional cigarettes. If the claim is approved, it would be a game changer for Altria and PMI. Not only would PM USA obtain a substantial marketing advantage over other RRPs, including e-cigarettes, but such a decision would also facilitate entries into other markets. The assessment, however, will take time. At the time of this writing, it was still unclear whether the FDA would even accept PMI’s application of 2 million-plus pages. Several MRTP applications have been filed with the FDA, but the agency has yet to sanction a modified-risk tobacco product.
Increasing capacity
Herzog is optimistic about the potential of iQOS. “Based on current success, we expect PMI to achieve critical mass—i.e., a 2 to 3 percent smoker share—within 12 to 24 months of entry into any given market, in line with PMI’s expectations,” she commented in an investor’s note. IQOS, she added, was resonating strongly with adult consumers. “Smoker conversion rates are exceptional across markets at 70 to 80 percent, and adult smoker awareness is growing—it now stands at 70 percent in Japan compared to 90 percent awareness of conventional Marlboro cigarettes.”
Favorable taxation of the consumables is expected to further contribute to the success of the product. At present, tax rates on HeatSticks are between 20 to 30 percent lower than those on combustible cigarettes, says Christopher R. Growe, managing director at Stifel Food and Tobacco Equity Research. “We believe this could add significantly to profitability for PMI, as the product tends to sell at the same retail price as the combustible products. We believe this opportunity represents upside to the company’s estimate of $720 million to $1.2 billion in annual profits from RRPs.”
More than 2 million adult smokers worldwide have already switched from combustible cigarettes to iQOS, PMI’s chief executive officer Andre Calantzopoulos said at the company’s annual shareholder meeting on May 3. In March, the company announced that it would invest approximately €300 million ($323.72 million) to convert its Papastratos cigarette factory in Greece into a manufacturing facility for the tobacco sticks to be used with iQOS. Located near Athens and expected to be fully operational by January 2018, the factory is expected to produce 20 billion units annually. PMI aims for an annual capacity of 100 billion units by the end of 2018.
The company also has a HeatSticks factory in Bologna, Italy, and a small-scale development center in Neuchatel, Switzerland.
The addition of production capacity will reduce per-unit production coast, according Herzog. She expects iQOS to generate $500 million in operating profit in 2018, increasing to around $15.5 billion by 2025.
Progress at all levels
In the meantime, PMI’s other RRP platforms are making progress, as well. Platform 2, to be commercialized under the name Teeps, is also an HnB product. Unlike iQOS, which heats tobacco using an electronically controlled blade, it features a carbon heat source that, when ignited, transfers heat to a patented tobacco plug.
Teeps is said to be more similar to a combustible cigarette than iQOS, which may make it an even more attractive alternative for smokers. It will also come with Marlboro-branded consumables and is scheduled for city tests and a possible national launch this year, according to Herzog.
Platforms 3 and 4 contain nicotine but no tobacco. Branded “Steem,” Platform 3 generates a nicotine-containing vapor using nicotine salt. When a consumer draws on the mouthpiece, a chemical reaction between nicotine—a weak base—and a weak organic acid takes place to produce a vapor containing nicotine salt. PMI acquired the Steem technology in 2011 from Jed Rose, of Duke University, and other inventors. The company expects to test the product in 2017.
PMI’s Platform 4 is called Mesh. It is a closed-system e-cigarette that features a new heating technology. Instead of using a wick and coil, as is common in most e-cigarettes, the Mesh atomizer has an embedded stainless steel mesh with more than 1,300 tiny holes in its cartridge. According to PMI, this increases the surface area in contact with the e-liquid and allows the e-liquid to be heated more precisely.
Mesh cartridges are manufactured, assembled, pre-filled and pre-sealed in a fully automated, Good Manufacturing Practices-compliant process in Europe, thus meeting strict regulatory requirements and addressing concerns of vapers regarding product quality, safety, consistency and origin. Mesh has been developed by Nicocigs, a U.K. vaping company that PMI acquired in 2014. The product is currently being tested in Birmingham, U.K.
Reflecting on the company’s progress toward a smoke-free future, 2016 was a pivotal year, according to Calantzopoulos. To date, PMI has hired more than 400 scientists and invested more than $3 billion in the research and development of RRPs.
The company has published the results of its scientific assessments of iQOS in more than 200 peer-reviewed publications and is talking with regulators around the world to make a case for its heat-not-burn technology.
To support its efforts, PMI has also increased transparency. In April, the company presented its Intervals website, an inhalation-toxicology repository for RRPs. The proof-of-concept data-sharing initiative hosts comprehensive, annotated data sets generated by PMI as part of its development and assessment of reduced-risk products.

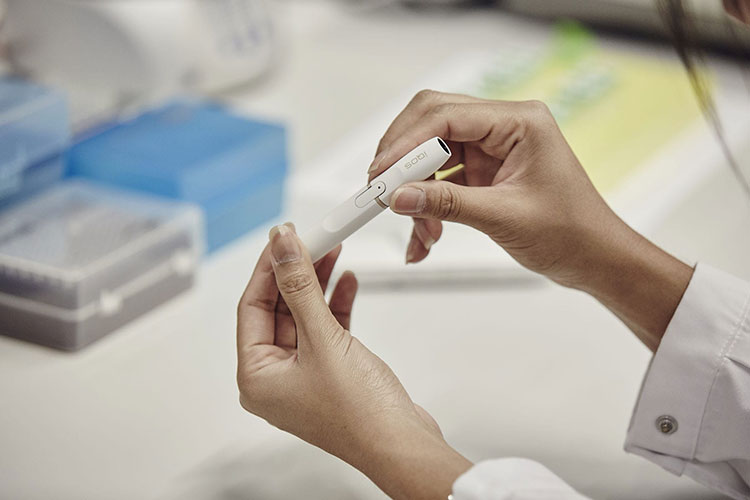




 Philip Morris International is unlikely to get permission to sell its iQOS heated tobacco device in Australia under current regulations, according to a story in the Sydney Morning Herald relayed by the TMA and quoting the assistant health minister, David Gillespie.
Philip Morris International is unlikely to get permission to sell its iQOS heated tobacco device in Australia under current regulations, according to a story in the Sydney Morning Herald relayed by the TMA and quoting the assistant health minister, David Gillespie.





