When tobacco heals instead of hurts
By Taco Tuinstra
The health consequences of tobacco use have been well documented. According to the World Health Organization, more than 8 million people worldwide succumb to tobacco-related illnesses, such as lung cancer, heart disease and chronic obstructive pulmonary disease, every year. For every person who dies due to tobacco, at least 30 people live with a serious tobacco-related illness, according to a 2019 policy brief by Tobacconomics.
Tobacco also carries a significant economic toll. A report prepared by the World Bank Group Global Tobacco Program expected the worldwide economic cost of smoking to reach $1.4 trillion in 2017, equivalent to 1.8 percent of the world’s gross domestic product that year. Little wonder, then, that many view the tobacco plant as evil.
Yet there is another, decidedly more benign, side to the Wicked Weed. When dried, set on fire and inhaled, the tobacco plant wreaks havoc; when deployed as a “green bioreactor,” the golden leaf has the potential to help address some of the world’s most vexing public health challenges, including, ironically, certain respiratory afflictions.

(Photo: Baiya Phytopharm).
Among the most striking examples of tobacco’s potential to heal rather than hurt are recent endeavors to develop an inoculation against the novel coronavirus. In February, Health Canada approved Covifenz, a tobacco plant-based Covid-19 vaccination developed by pharmaceutical giant GlaxoSmithKline and Medicago, a biopharmaceutical company backed by Philip Morris International. According to Health Canada, Covifenz is the world’s first vaccine approved for human use that utilizes a plant-based protein technology.
As it turns out, plants—and especially tobacco plants (see sidebar)—lend themselves exceptionally well to developing pharmaceutical compounds. In many ways, they are better suited to the task than the man-made bioreactors used in traditional vaccine development.
To create a vaccine, scientists must produce antigens—molecules that trigger an immune response to a specific virus or bacteria. Antigens for conventional vaccines are made by infecting cells from insects, monkeys, hamsters or other sources in the laboratory with a virus or a bit of viral genetic code that tricks the cells into making copies of the virus or antigen. The cells incubate in bioreactors for extended periods of time and then undergo a complex purification process before being packaged into vials.
Bioreactors are expensive, however, and their operation requires trained personnel. They are also susceptible to contamination, forcing vaccine developers to keep bioreactors growing different types of antigens far apart and under sterile conditions.

Plants are natural bioreactors, according to Dahlia Garwe, the former CEO of the Kutsaga Tobacco Research Station in Zimbabwe. “They are able to perform eukaryotic post-translational modifications that are often essential for biological activity of many mammalian proteins,” she explains. When infected with DNA from Covid-19 or other viruses, plant cells will make millions of copies of virus-like particles that can serve as antigens without being infectious.
Using plants instead of mechanical bioreactors offers many benefits, notes Garwe. Plants are cheap to grow, easy to manipulate and resistant to contaminations that could present problems for humans. “Green bioreactors do not suffer the same risk of pathogen contamination as seen in mammalian cell culture as there are no known cross-kingdom pathogens,” she says.
With light as their primary energy source, plants are less expensive to work with than traditional cell culture systems, allowing for inexpensive and nearly unlimited scalability. Because plant systems are robust and inert, they are also easier to handle and purify than other systems.
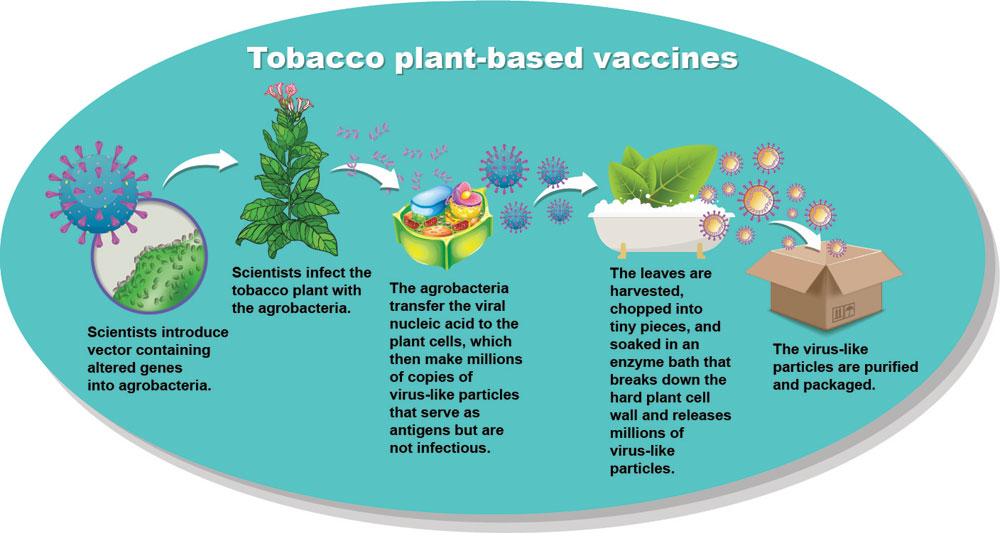
Medicago is not the only company that has grasped the promise of plants in the development of vaccines and therapeutics. At least three other organizations are using Nicotiana benthamiana, a close relative of the tobacco plant used in cigarettes, to develop Covid-19 vaccines. In December 2020, the U.S. Food and Drug Administration approved an investigational new drug application for a Covid-19 vaccine by BAT subsidiary Kentucky Bioprocessing (KBP, now part of KBio). The company is currently conducting Phase I clinical trials, which are the first steps in testing the safety and side effects of a pharmaceutical in humans.
According to BAT, plant-based vaccines have several advantages over serums developed with conventional technologies, including speed and thermostability. Whereas traditional methods can take months to yield the desired vaccine ingredients, KBP’s tobacco plants do so within six weeks. And unlike some of the existing Covid-19 vaccines, KBP’s vaccine candidate has the potential to be stable at room temperature, an important benefit, especially for public health networks in countries with warm climates and few refrigerated trucks and warehouses.
Fearing supply constraints and “vaccine nationalism” at the start of the pandemic, scientists in Thailand have joined the race as well. Baiya Phytopharm, a plant-produced biologics company, is working with tobacco in part because that crop can be easily and inexpensively cultivated domestically, thus reducing Thailand’s reliance on foreign-made vaccines. According to the Bangkok Post, Thailand has more than 10,000 tobacco growers.

In an email exchange, Baiya Phytopharm co-founder Waranyoo Phoolcharoen told Tobacco Reporter that the company had finished Phase 1 clinical trials and was happy with the data. Baiya Phytopharm is currently developing a new generation of its vaccine for the omicron variant of Covid-19 and expects to conduct Phase 1 trials this month (April). If the vaccine candidate does well in subsequent trials, it could be available in Thailand by the end of the year, according to Phoolcharoen.
Meanwhile, Akdeniz University in Turkey is using a protein produced by tobacco plants to develop a Covid-19 vaccine. Its technology is based on an angiotensin-converting enzyme 2 and can be administered as an injection or through a spray. According to an April 2021 report in Hurriyet Daily News, tests on mice have demonstrated a high level of inhibition of the Covid-19 virus from entering the cell. At the time of publication, Akdeniz University was seeking funding to conduct clinical trials in humans.
In addition to Covid-19, scientists have sought to tackle many other diseases with the help of tobacco. In response to concerns about bioterrorism following the September 11 attacks in the United States, researchers at the University of Central Florida used tobacco plants to create a protective antigen against anthrax. According to an article published in Environmental Health Perspectives, mice immunized with the agent survived anthrax injections of 1.5 times the deadly dose.
In 2014, KBP, working in cooperation with the U.S. Biomedical Advanced Research and Development Authority, developed, tested and used ZMapp, an antibody cocktail to treat infection from the Ebola virus, which was raging in west Africa at the time. In under six weeks, KBP completed cGMP manufacturing of each of the three monoclonal antibodies comprising the ZMapp cocktail, which received temporary emergency authorization from the FDA.
Pushing the envelope further still, Collplant of Israel is using tobacco to generate human tissue and organs. Collplant’s technology allows for the production of large quantities of medical grade human collagen, which is a primary building block for the human body and essential for tissue repair. Physicians use collagen products to treat chronic wounds, burns and other afflictions. Deriving collagen from tobacco rather than from traditional sources, such as cadavers, cows or pigs, offers several advantages, including stable fibrillates, pure molecular structures and no immunogenic responses, according to Collplant.
While the endeavors described above are only a sample of the many ways in which tobacco is deployed to produce pharmaceutical and other useful compounds, they vividly illustrate the potential of the golden leaf to help solve rather than create problems—a side that is often overlooked due to the health toll extracted by smoking.
The Green Bioreactor
 While despised in the court of public opinion, tobacco is highly valued among plant scientists.
While despised in the court of public opinion, tobacco is highly valued among plant scientists.
For starters, the species has good physiology for research. “Seeds are easily sterilized, and germination is very quick, so generating experimental material is easy,” says David Norman, senior scientist at Demeetra AgBio, a company that is using gene editing for crop trait development and that also works with tobacco (see “Genetic Scissors,” Tobacco Reporter, August 2021). “Tobacco grows fairly fast compared to many plants, to where flowering and mature seeds can be attained in just a couple months.”
The soft tobacco leaf is ideal for transfection, allowing researchers to easily introduce DNA or RNA into its cells. What’s more, regeneration mechanisms from callus in tobacco are extremely efficient. In addition to allowing genetic modifications on leaf material, tobacco is also easily propagated as a cell culture or protoplast culture.
The fact that tobacco is neither a food nor a feed crop makes it even more attractive as a plant for conducting research, according to Dahlia Garwe, the former CEO of the Kutsaga Tobacco Research Station in Zimbabwe. Using tobacco, she says, reduces the likelihood of transgenic material contaminating food or feed supply chains—an important consideration when many consumers remain wary of genetically manipulated foods.
Tobacco is also well understood from a genetic engineering and molecular biology standpoint; it has been more characterized than any other plant system. Scientists have been researching and genetically modifying tobacco since the early 20th century.
Meanwhile, biochemists have been able to produce many recombinant proteins in tobacco, with the plants serving as bioreactors for largescale production of pharmaceutical and industrial compounds. “Tobacco can easily take in DNA to produce these compounds when injected into the leaf or through vacuum infiltration of whole plants,” says Norman.
The vacuum infiltration method, in particular, can be scaled up to infect many plants at one time, according to Norman. “After infiltration, tobacco transiently expresses the DNA introduced for a short while and makes the protein of interest in the leaves.
“While the protein production in plants may not be that impressive on a per plant scale, up to a few percent depending on the protein of interest, you have to consider the yield per acre for tobacco. On an average acre of tobacco, the yield can be several tons of leaf material. With that scale of leaf material per acre, suddenly those small protein yields add up to being significant and potentially economically viable.”–T.T.
The Plant-Based Vaccine: A Late Bloomer?
In February, Medicago’s Covifenz Covid-19 vaccine received the green light from Health Canada, making it the world’s first plant-based vaccine approved for human use. Considering how long scientists have known about the potential of plant-based vaccines—the proof of concept dates back some 30 years—it may seem surprising that the first regulatory approval for such a product was granted only this year.
While the U.S. Department of Agriculture in 2006 gave the thumbs-up to a plant-derived vaccine to inoculate chickens against the lethal Newcastle disease, most of the projects targeted at human vaccines never even made it to end-stage clinical trials. The FDA did approve Protalix’s plant-based drug to treat Gaucher’s disease in humans in 2012 and in 2014 gave emergency authorization to ZMapp, a plant-based biological medicine manufactured by KBP (now part of KBio) to treat Ebola, but the first was not a vaccine, and the second was approved under special conditions.
Dahlia Garwe, the former CEO of the Kutsaga Tobacco Research Board in Zimbabwe, suspects the “delay” in approving plant-based vaccines is due to regulatory issues and public perceptions of genetically modified organisms (GMOs). “Most vaccines produced in plants would most likely be classified as GMOs, which automatically makes them ‘undesirables,’” she ventures.
Kathleen Hefferon, who teaches microbiology at Cornell University, acknowledges that public perception may be part of the problem but believes that many consumers would accept plant-made vaccines just as they have accepted vaccines produced in eggs and mammalian cells. “They are familiar with the GM technology when used for medical purposes,” she says. “It is when it comes to genetic modification in food products that some people seem to raise concerns.”
Hefferon suspects that the absence of a clear regulatory path represents the greatest obstacle as it may have made pharmaceutical companies wary of investing in this technology. The carrot suspension cell culture that Protalix used for its Gaucher’s disease drug somewhat resembles Chinese hamster ovary cell culture, she notes. “That made it a little easier for regulators.”
Patrick Doyle, CEO of KBio Holdings, a company set up by BAT to accelerate the development and production of novel treatments with plant-based technology, says it is important to remember that in a normal R&D cycle, medicines and vaccines on average take over 10 years, even when using a proven technology or approach.
“In fact, as a result of the global health crisis presented by Covid[-19], development, regulatory review and approval timelines were accelerated to support the delivery of much-needed vaccines,” he says. “Before gaining approval for any vaccine, manufacturers must generate sufficient data to demonstrate the efficacy and safety of the candidate and technology, which takes time. For example, the mRNA platform, first discovered in the early 1960s, was a genetic technology that had long held huge promise but only received the first FDA [Food and Drug Administration] emergency use authorization for the prevention of Covid-19 disease in individuals 16 years of age and older on Dec. 20.”
According to Doyle, the positive data generated and the subsequent approval of Medicago’s vaccine is both a positive in terms of creating another approved treatment option for Covid-19 and reinforcing the potential of plant-based vaccines.
Hefferon agrees that Medicago’s success could set the stage for plant-made pharmaceutical commercialization in future years, and her optimism appears to be shared by others. Research and Markets expects the value of the global plant-based vaccines market to reach $2.62 billion by 2028—more than double the 2021 figure. Tobacco companies seem well attuned to the potential as evidenced by PMI’s investment in Medicago and by BAT’s creation of KBio. Doyle says KBio will explore new opportunities to develop its plant-based production system, which has the potential to offer greater speed, thermostability and scale-up opportunity and.
Hefferon says she’s very happy about the growing momentum for plant-based vaccines. “Too bad it took a pandemic to make it happen.” –T.T.
Uphill Battle: Tobacco-Backed Vaccines Continue To Face Skepticism From Health Authorities
While tobacco plants lend themselves exceptionally well to the creation of pharmaceutical compounds, vaccinations and treatments developed by tobacco-backed organizations still face an uphill battle as demonstrated by recent World Health Organization actions.
During a March 16 media briefing, the WHO’s assistant director-general for drug access, vaccines and pharmaceuticals, Mariangela Simao, said the global health body had paused the process for pre-qualification of Medicago’s new Covifenz Covid-19 shot due to the biopharmaceutical firm’s link to Philip Morris International, which owns about one-third of the Canadian company.
“The WHO and the U.N. have a very strict policy regarding engagement with the tobacco and arms [industries], so it’s very likely it won’t be accepted for emergency use listing,” said Simao. The WHO persists in its opposition despite Health Canada’s February approval of Covifenz for adults between the ages of 18 and 64.
In a statement published by the CBC, Medicago said it believes authorization decisions should be based on the quality, efficiency and safety of the vaccine, not who owns shares in the manufacturer.
“It is our understanding that the WHO has made a decision to pause the approval of the vaccine and that this decision is related to Medicago’s minority shareholder and not to the efficacy and safety of the vaccine, which was demonstrated with the approval by Health Canada,” the statement reads.
Derek Yach, a global health consultant, was aghast by the WHO’s suggestion that it might reject Medicago’s vaccine based on the company’s relationship with PMI.
“‘Pikuach nefesh’ is the ethical principle in Jewish law that the preservation of human life overrides virtually any other religious rule,” he said. “Most other religions support a variant of this. WHO violates this ethical principle when it denies people access to a lifesaving vaccine.”
If the WHO follows through, the vaccine would be the first Western-manufactured Covid-19 shot to be rejected by the global health body, according to Bloomberg. —T.T.