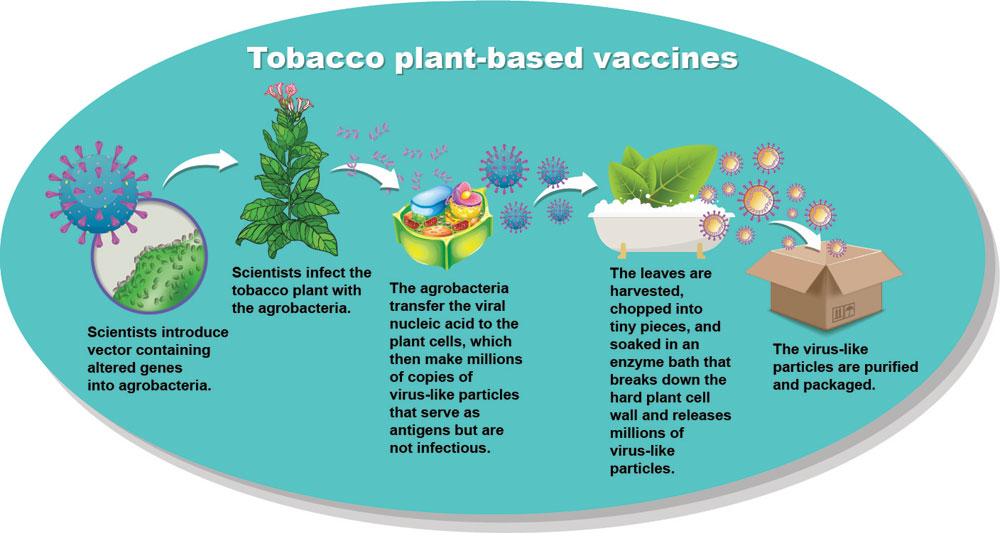
In February, Medicago’s Covifenz Covid-19 vaccine received the green light from Health Canada, making it the world’s first plant-based vaccine approved for human use. Considering how long scientists have known about the potential of plant-based vaccines—the proof of concept dates back some 30 years—it may seem surprising that the first regulatory approval for such a product was granted only this year.
While the U.S. Department of Agriculture in 2006 gave the thumbs-up to a plant-derived vaccine to inoculate chickens against the lethal Newcastle disease, most of the projects targeted at human vaccines never even made it to end-stage clinical trials. The FDA did approve Protalix’s plant-based drug to treat Gaucher’s disease in humans in 2012 and in 2014 gave emergency authorization to ZMapp, a plant-based biological medicine manufactured by KBP (now part of KBio) to treat Ebola, but the first was not a vaccine, and the second was approved under special conditions.
Dahlia Garwe, the former CEO of the Kutsaga Tobacco Research Board in Zimbabwe, suspects the “delay” in approving plant-based vaccines is due to regulatory issues and public perceptions of genetically modified organisms (GMOs). “Most vaccines produced in plants would most likely be classified as GMOs, which automatically makes them ‘undesirables,’” she ventures.
Kathleen Hefferon, who teaches microbiology at Cornell University, acknowledges that public perception may be part of the problem but believes that many consumers would accept plant-made vaccines just as they have accepted vaccines produced in eggs and mammalian cells. “They are familiar with the GM technology when used for medical purposes,” she says. “It is when it comes to genetic modification in food products that some people seem to raise concerns.”
Hefferon suspects that the absence of a clear regulatory path represents the greatest obstacle as it may have made pharmaceutical companies wary of investing in this technology. The carrot suspension cell culture that Protalix used for its Gaucher’s disease drug somewhat resembles Chinese hamster ovary cell culture, she notes. “That made it a little easier for regulators.”
Patrick Doyle, CEO of KBio Holdings, a company set up by BAT to accelerate the development and production of novel treatments with plant-based technology, says it is important to remember that in a normal R&D cycle, medicines and vaccines on average take over 10 years, even when using a proven technology or approach.
“In fact, as a result of the global health crisis presented by Covid[-19], development, regulatory review and approval timelines were accelerated to support the delivery of much-needed vaccines,” he says. “Before gaining approval for any vaccine, manufacturers must generate sufficient data to demonstrate the efficacy and safety of the candidate and technology, which takes time. For example, the mRNA platform, first discovered in the early 1960s, was a genetic technology that had long held huge promise but only received the first FDA [Food and Drug Administration] emergency use authorization for the prevention of Covid-19 disease in individuals 16 years of age and older on Dec. 20.”
According to Doyle, the positive data generated and the subsequent approval of Medicago’s vaccine is both a positive in terms of creating another approved treatment option for Covid-19 and reinforcing the potential of plant-based vaccines.
Hefferon agrees that Medicago’s success could set the stage for plant-made pharmaceutical commercialization in future years, and her optimism appears to be shared by others. Research and Markets expects the value of the global plant-based vaccines market to reach $2.62 billion by 2028—more than double the 2021 figure. Tobacco companies seem well attuned to the potential as evidenced by PMI’s investment in Medicago and by BAT’s creation of KBio. Doyle says KBio will explore new opportunities to develop its plant-based production system, which has the potential to offer greater speed, thermostability and scale-up opportunity and.
Hefferon says she’s very happy about the growing momentum for plant-based vaccines. “Too bad it took a pandemic to make it happen.” –T.T.